A trivalent mucosal vaccine encoding phylogenetically inferred ancestral RBD sequences confers pan-Sarbecovirus protection in mice
IF 20.6
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
The continued emergence of SARS-CoV-2 variants and the threat of future Sarbecovirus zoonoses have spurred the design of vaccines that can induce broad immunity against multiple coronaviruses. Here, we use computational methods to infer ancestral phylogenetic reconstructions of receptor binding domain (RBD) sequences across multiple Sarbecovirus clades and incorporate them into a multivalent adenoviral-vectored vaccine. Mice immunized with this pan-Sarbecovirus vaccine are protected in the upper and lower respiratory tracts against infection by historical and contemporary SARS-CoV-2 variants, SARS-CoV, and pre-emergent SHC014 and Pangolin/GD coronavirus strains. Using genetic and immunological approaches, we demonstrate that vaccine-induced protection unexpectedly is conferred principally by CD4+ and CD8+ T cell-mediated anamnestic responses. Importantly, prior mRNA vaccination or SARS-CoV-2 respiratory infection does not alter the efficacy of the mucosally delivered pan-Sarbecovirus vaccine. These data highlight the promise of a phylogenetic approach for antigen and vaccine design against existing and pre-emergent Sarbecoviruses with pandemic potential.
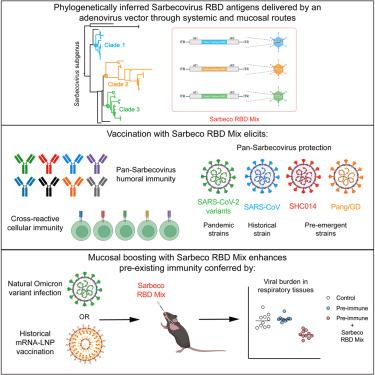
编码系统发育推断出的祖先 RBD 序列的三价粘膜疫苗可为小鼠提供泛沙巴病毒保护
SARS-CoV-2 变异体的不断出现以及未来 Sarbecovirus 人畜共患病的威胁促使人们设计能诱导多种冠状病毒广泛免疫的疫苗。在本文中,我们使用计算方法推断了多个沙棘病毒支系的受体结合域(RBD)序列的祖先系统发育重建,并将其纳入多价腺病毒载体疫苗中。小鼠接种这种泛沙士病毒疫苗后,上呼吸道和下呼吸道均可免受历史和当代 SARS-CoV-2 变体、SARS-CoV 以及 SHC014 和 Pangolin/GD 冠状病毒毒株的感染。我们利用遗传学和免疫学方法证明,疫苗诱导的保护作用出乎意料地主要由 CD4+ 和 CD8+ T 细胞介导的过敏反应产生。重要的是,之前的 mRNA 疫苗接种或 SARS-CoV-2 呼吸道感染不会改变粘膜递送的泛沙士病毒疫苗的功效。这些数据凸显了系统发育方法在抗原和疫苗设计方面的前景,这种方法可用于抗击现有的和爆发前的具有大流行潜力的沙士病毒。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


