Polyoxometalate oxate solution: An electrolyte additive to sustainably improve the anodes electrode of aqueous Zn ion batteries
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Aqueous zinc-ion batteries (AZIBs) are favored by researchers because of their high safety performance and abundant zinc resources. In this work, low-cost was selected as the electrolyte additive to continuously improve the problems faced by the anode. Molybdenum-based solution can be reduced on the surface of zinc anode to form a uniform protective layer, and polyoxometalate can disperse metal elements more evenly, so that Zn2+ deposition is more uniform and AZIBs has more stable cycling performance. The symmetric battery assembled after adding Polyoxometalate oxate solution can maintain a stable potential of more than 1900 h at 5mA cm−2 and 1mAh cm−2, which is three times the cycle time of the battery assembled with ZnSO4 as the electrolyte (600 h). In addition, Mo has a certain anti-corrosion effect, which can prevent the corrosion reaction on the surface of zinc anode. The Tafel diagram shows that the corrosion current decreased from 1.995 mA cm−2 to 1.584 mA cm−2 after the addition of polyoxometalate oxate solution. The contrast effect is more obvious in SEM images. Different from the traditional zinc anode surface optimization, the addition of polyoxometalate oxate solution can continuously form auxiliary nucleation sites on the negative electrode surface to make Zn2+ deposition more uniform. When Na doped vanadium dioxide was used as the cathode to form full battery, the specific capacity could still reach 143.73mAh g−1 after 1000 cycles at the current density of 2 A/g. In this study, adding low concentration of polyoxometalate oxate solution to the electrolyte can continuously generate a stable protective layer during the battery operation, providing a feasible scheme for continuous improvement of AZIBs zinc anode.
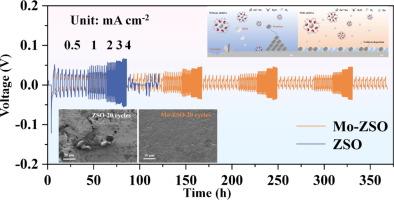
聚氧化金属氧化物溶液:一种可持续改善锌离子水电池阳极电极的电解质添加剂
锌离子水电池(AZIBs)因其较高的安全性能和丰富的锌资源而受到研究人员的青睐。本研究选择低成本作为电解质添加剂,以持续改善阳极所面临的问题。钼基溶液能在锌阳极表面还原形成均匀的保护层,聚氧化铝能更均匀地分散金属元素,使 Zn2+ 沉积更均匀,AZIBs 的循环性能更稳定。加入聚氧化金属氧化物溶液后组装的对称电池在 5mA cm-2 和 1mAh cm-2 的条件下可保持 1900 小时以上的稳定电位,是以 ZnSO4 为电解液组装的电池循环时间(600 小时)的三倍。此外,钼还具有一定的抗腐蚀作用,可以防止锌阳极表面的腐蚀反应。从 Tafel 图中可以看出,加入聚氧化金属氧化物溶液后,腐蚀电流从 1.995 mA cm-2 降至 1.584 mA cm-2。在扫描电镜图像中,对比效果更为明显。与传统的锌阳极表面优化不同,添加多氧化物金属氧化物溶液可以在负极表面不断形成辅助成核点,使 Zn2+ 沉积更加均匀。当使用掺杂 Na 的二氧化钒作为正极形成全电池时,在电流密度为 2 A/g 的条件下,循环 1000 次后比容量仍能达到 143.73mAh g-1。在这项研究中,向电解液中添加低浓度的聚氧化金属氧化物溶液可在电池运行过程中持续生成稳定的保护层,为持续改进 AZIBs 锌负极提供了可行的方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


