Low dose exposure to dioxins alters hepatic energy metabolism and steatotic liver disease development in a sex-specific manner
IF 10.3
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
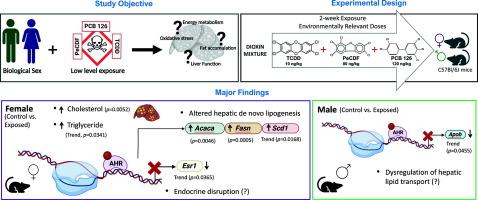
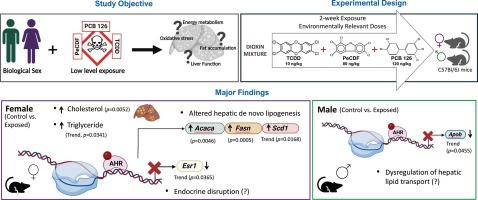
“Dioxins” are persistent organic pollutants (POPs) that are continuously present in the environment at appreciable levels and have been associated with increased risk of steatotic liver disease (SLD). However, current understanding of the role of sex and effects of mixtures of dioxins in SLD development is limited. Additionally, there exists debates on the levels of dioxins required to be considered dangerous as emphasis has shifted from high level exposure events to the steady state of lower-level exposures. We therefore investigated sex-dependent effects of low-level exposures to a mixture of dioxins: 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD), 2,3,4,7,8-Pentachlorodibenzofuran (PeCDF) and Polychlorinated biphenyl 126 (PCB126), in the context of SLD and associated metabolic dysfunction. Male and female C57BL/6J mice were fed a low-fat diet and weekly administered either vehicle control or TCDD (10 ng/kg), PeCDF (80 ng/kg) and PCB 126 (140 ng/kg) over a two-week period. Female mice generally demonstrated higher hepatic fat content compared to males. However, exposure to dioxins further elevated hepatic cholesterol levels in females, and this was accompanied by increased lipogenic gene expression (Acaca, Fasn) in the liver. In contrast, exposed males but not females displayed higher white adipose tissue weights. Furthermore, TCDD + PeCDF + PCB126 activated the AHR (hepatic Cyp1a1, Cyp1a2 induction); with Cyp1a1 induction observed only in exposed females. Notably, gene expression of hepatic albumin (Alb) was also reduced only in exposed females. Overall, exposure to the low dose dioxin mixture compromised hepatic homeostasis via metabolic perturbations, and hepatic dysregulation was more accelerated in female livers.
低剂量暴露于二恶英会以性别特异性方式改变肝脏能量代谢和脂肪性肝病的发展
"二恶英 "是一种持久性有机污染物(POPs),持续存在于环境中,且含量可观,与脂肪肝(SLD)风险增加有关。然而,目前人们对二恶英混合物在脂肪肝发病过程中的性别作用和影响的了解还很有限。此外,由于重点已从高浓度暴露事件转移到低浓度暴露的稳定状态,因此对于二恶英的危险水平还存在争议。因此,我们研究了低水平暴露于二恶英混合物(2,3,7,8-四氯二苯并对二恶英(TCDD)、2,3,4,7,8-五氯二苯并呋喃(PeCDF)和多氯联苯 126(PCB126))对SLD和相关代谢功能障碍的性别依赖性影响。雄性和雌性 C57BL/6J 小鼠均以低脂饮食为食,并在两周内每周服用一次对照药物或 TCDD(10 纳克/千克)、PeCDF(80 纳克/千克)和 PCB 126(140 纳克/千克)。与雄性小鼠相比,雌性小鼠的肝脏脂肪含量普遍较高。然而,暴露于二恶英会进一步提高雌性小鼠肝脏中的胆固醇水平,同时肝脏中的致脂基因(Acaca、Fasn)表达也会增加。与此相反,暴露于二恶英的雄性动物(而非雌性动物)显示出更高的白色脂肪组织重量。此外,TCDD + PeCDF + PCB126 激活了 AHR(肝脏 Cyp1a1、Cyp1a2 诱导);仅在暴露的雌性中观察到 Cyp1a1 诱导。值得注意的是,肝脏白蛋白(Alb)的基因表达也仅在暴露的雌性中减少。总之,暴露于低剂量二恶英混合物会通过新陈代谢扰动损害肝脏稳态,而雌性肝脏的肝功能失调速度更快。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environment International
环境科学-环境科学
CiteScore
21.90
自引率
3.40%
发文量
734
审稿时长
2.8 months
期刊介绍:
Environmental Health publishes manuscripts focusing on critical aspects of environmental and occupational medicine, including studies in toxicology and epidemiology, to illuminate the human health implications of exposure to environmental hazards. The journal adopts an open-access model and practices open peer review.
It caters to scientists and practitioners across all environmental science domains, directly or indirectly impacting human health and well-being. With a commitment to enhancing the prevention of environmentally-related health risks, Environmental Health serves as a public health journal for the community and scientists engaged in matters of public health significance concerning the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


