Development of heterostructured ZnCo2O4@Ni-MOF electrode for the asymmetric supercapacitor and electrocatalytic oxygen evolution reaction applications
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
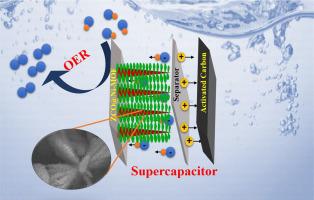
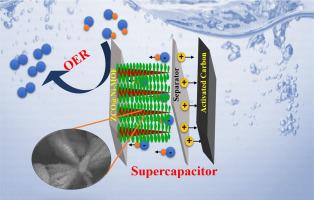
The stable structure and material combination design significantly improve the performance of electrochemical energy storage and water splitting. In the present study, we developed a ZCO@Ni-MOF core-shell structure over a nickel foam electrode, which is synthesized through a two-step hydrothermal treatment. The developed material is comprehensively analyzed to confirm structural, chemical, electronic, surface, and morphological characteristics using X-ray diffractometer (XRD), X-ray photoelectron spectroscopy (XPS), attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopy, scanning electron microscope (SEM), and transmission electron microscope (TEM). Electrochemical investigations using a three-electrode system revealed that ZCO@Ni-MOF demonstrated an impressive specific capacitance of 1800 F g−1 at a current density of 2 A g−1 in a 1 M KOH electrolyte. The electrochemical findings are consistent across various electrochemical techniques. Furthermore, in-depth studies regarding p-n junction formation, interlayer spacing, and reaction kinetics studies are briefly analyzed with Mott-Schottky, Ex-situ XRD, and operando impedance studies. Moreover, an asymmetric supercapacitor (ASC) is assembled with ZCO@Ni-MOF as the positive electrode and activated carbon as the negative electrode in a Swagelok cell. This configuration demonstrated an energy density of 13.6 Wh kg−1 at a power density of 225 W kg−1. The ASC exhibited performance by retaining 91 % of its initial capacity even after 1500 cycles. For practical demonstration, two ASCs are fabricated and assembled in series to light up an LED, and the light-up duration is analyzed. For the oxygen evolution reaction (OER) study, the ZCO@Ni-MOF-based electrode exhibited activity with a lower overpotential of 340 mV (50 mA cm−2) in an alkaline environment and was responsible for stability for about 10 h. This combination reiterates the promising material aspects in energy storage and conversion devices, instilling hope for its potential applications.
开发用于不对称超级电容器和电催化氧进化反应的异质结构 ZnCo2O4@Ni-MOF 电极
稳定的结构和材料组合设计大大提高了电化学储能和水分离的性能。在本研究中,我们在泡沫镍电极上开发了一种 ZCO@Ni-MOF 核壳结构,该结构是通过两步水热处理合成的。利用 X 射线衍射仪 (XRD)、X 射线光电子能谱 (XPS)、衰减全反射-傅立叶变换红外光谱 (ATR-FTIR)、扫描电子显微镜 (SEM) 和透射电子显微镜 (TEM) 对所开发的材料进行了全面分析,以确认其结构、化学、电子、表面和形态特征。使用三电极系统进行的电化学研究表明,ZCO@Ni-MOF 在 1 M KOH 电解液中的电流密度为 2 A g-1 时,比电容高达 1800 F g-1。这些电化学研究结果在各种电化学技术中都是一致的。此外,还通过莫特-肖特基、原位 XRD 和操作阻抗研究对 p-n 结的形成、层间距和反应动力学研究进行了深入分析。此外,在一个世伟洛克电池中,以 ZCO@Ni-MOF 为正极,以活性炭为负极,组装了一个不对称超级电容器(ASC)。这种配置在功率密度为 225 W kg-1 时的能量密度为 13.6 Wh kg-1。ASC 的性能表现为,即使经过 1500 次循环,其初始容量仍能保持 91%。为了进行实际演示,我们制作了两个 ASC,并将其串联起来点亮一个 LED,同时对点亮持续时间进行了分析。在氧进化反应(OER)研究中,基于 ZCO@Ni-MOF 的电极在碱性环境中表现出较低的活性,过电位为 340 mV (50 mA cm-2),并能稳定工作约 10 h。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


