Facile Synthesis of Tungsten Diboride (WB2-191) in Molten Salt and Its Hydrogen Evolution Performance in Acidic and Alkaline Media
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The AlB2-type WB2-191 with only graphene-like flat boron layers is deemed to be an ideal electrocatalyst for hydrogen evolution. However, such investigation is severely hindered by the lack of facile synthesis methods for this type of WB2-191. Herein, we report the successful synthesis of AlB2-type WB2-191 by a one-step molten salt method via the reaction of WCl6 and NaBH4 in LiCl–KCl molten salt at 800 °C under atmospheric pressure. The as-synthesized WB2-191 presents a nanosheet structure, as demonstrated by the scanning electron microscope and transmission electron microscopy results with a large BET surface area of 63 m2/g. Such WB2-191 nanosheets exhibit remarkable hydrogen evolution reaction activity, delivering low overpotentials of 121 in 0.5 H2SO4 and 147 mV in 1.0 M KOH to drive 10 mA/cm2, which is superior to any previously reported binary tungsten borides. Furthermore, an outstanding stability was shown over 70 h of potentiostatic operations in both acidic and alkaline electrolytes.
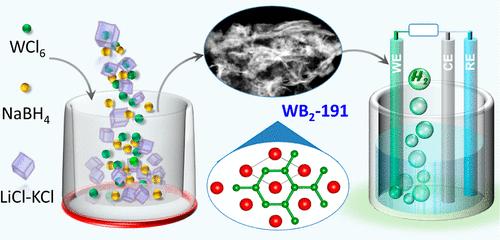
熔盐中二硼化钨 (WB2-191) 的简易合成及其在酸性和碱性介质中的氢演化性能
只有石墨烯状扁平硼层的 AlB2 型 WB2-191 被认为是理想的氢进化电催化剂。然而,由于缺乏这类 WB2-191 的简便合成方法,这种研究受到严重阻碍。在此,我们报告了在 800 °C 常压下,通过 WCl6 和 NaBH4 在 LiCl-KCl 熔盐中的反应,采用一步熔盐法成功合成了 AlB2 型 WB2-191。扫描电子显微镜和透射电子显微镜结果表明,合成的 WB2-191 呈纳米片状结构,具有 63 m2/g 的大 BET 表面积。这种 WB2-191 纳米片具有显著的氢进化反应活性,在 0.5 H2SO4 和 1.0 M KOH 中分别产生 121 和 147 mV 的低过电位,可驱动 10 mA/cm2,优于之前报道的任何二元硼化钨。此外,在酸性和碱性电解质中进行 70 小时的静电电位操作也显示出卓越的稳定性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


