Glucose electrooxidation on carbon supported NiAu electrocatalysts
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
NiAu/C nanomaterials are synthesised using a wet chemistry method with targeted Au atomic ratios of 10%, 20% and 30%. Physicochemical characterisations indicate that the materials have mean compositions close to the nominal ones but ca. 20 at% Au richer in average than expected (Au ratios of 13.6 at%, 23.1 at% and 35.9 at%, respectively). The NiAu/C materials are composed of Au-rich spherical-like Janus particles of several tenths nm and of a phase of very small Ni-rich nanoparticles and Ni(OH2) clusters. The electrochemical measurements in a 0.1 M NaOH/0.1 M glucose electrolyte indicate that the NiAu20/C catalyst is the most active for the glucose oxidation reaction, leading to a mass activity at +0.6 V vs RHE more than 1.5 times higher than that with a pure Au/C catalyst, although the Au content is almost 5 times lower. The chronoamperometry measurements for 900 s at +0.6 V vs RHE confirm the activity gain with the NiAu20/C catalyst. The electrolysis measurement at a cell voltage of +Z+0.6 V for 6 hours shows that the NiAu20/C catalyst is selective towards the production of gluconic acid, with a faradaic efficiency higher than 100%, indicating the occurrence of a 1-electron reaction with anodic hydrogen coproduction. At +0.8 V, the faradaic efficiency is lower than 100 %, indicating the formation of other products than gluconic acid, but at a very low extent (not detectable by HPLC) guarantying a very high selectivity towards gluconic acid.
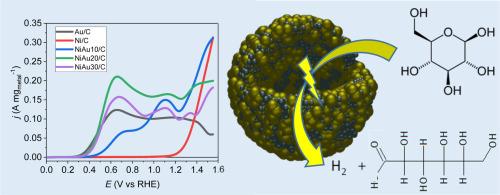
碳支撑 NiAu 电催化剂上的葡萄糖电氧化作用
采用湿化学方法合成了 NiAu/C 纳米材料,目标金原子比分别为 10%、20% 和 30%。理化特性表明,这些材料的平均成分接近标称成分,但平均金含量比预期高出约 20 个百分点(金比率分别为 13.6 个百分点、23.1 个百分点和 35.9 个百分点)。NiAu/C 材料由万分之几纳米的富金球状 Janus 颗粒和极小的富镍纳米颗粒及 Ni(OH2)团簇组成。在 0.1 M NaOH/0.1 M 葡萄糖电解液中进行的电化学测量表明,NiAu20/C 催化剂在葡萄糖氧化反应中最为活跃,在 +0.6 V 对比 RHE 时的质量活性比纯 Au/C 催化剂高出 1.5 倍以上,尽管 Au 的含量几乎低了 5 倍。在 +0.6 V 对 RHE 条件下进行的 900 秒计时器测量证实了 NiAu20/C 催化剂的活性提高。在 +Z+0.6 V 的电池电压下进行 6 小时的电解测量表明,NiAu20/C 催化剂对葡萄糖酸的生产具有选择性,其远红外效率高于 100%,这表明发生了阳极氢气共生的 1 电子反应。在 +0.8 V 电压下,法拉第效率低于 100%,这表明除葡萄糖酸外,还生成了其他产物,但生成量极低(HPLC 检测不到),保证了对葡萄糖酸的极高选择性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


