Pitfall on the interpretation of double layer capacitance increase after accelerated stress test of hydrogen evolution reaction on NiMo catalysts
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
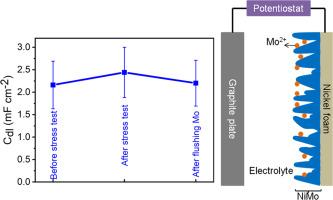
Critical investigation of methods used to screen the catalytic activity of different electrocatalysts is crucial for the development of water electrolysis technology. One of such protocols to justify the catalytic activity trend among different catalysts involves determining their electrochemically active surface area (ECSA) which is usually estimated from non-Faradic double layered capacitance (Cdl) of the catalyst material. Furthermore, catalytic current normalized with ECSA is frequently used to gain insight into the intrinsic activity of a catalyst. Since not all the metallic catalysts are highly stable under the electrolysis conditions, it is highly important, though rarely explored, to investigate whether or not the adsorption/desorption of leached/dissolved multivalent metal ions influences the measurement of non-Faradic Cdl. Here, we explore the possible influence of Mo leached from NiMo alloy on the measured Cdl of NiMo. Using cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS), Cdl of NiMo alloy was measured before and after carrying out long term (chronopotentiometric and CV cycling) hydrogen evolution reaction (HER) in alkaline conditions. After extended HER testing, we observe a notable rise (∼22% averaging all our experiments) in the Cdl of NiMo alloy when measured with traditional methods. Interestingly, only 8% of this increase can be attributed to an expansion in surface area. We hypothesize that the majority of the increase in Cdl stems from the higher amount of charges stored through leached multivalent ions, which accumulate within surface cavities.
解读镍钼催化剂氢进化反应加速应力测试后双层电容增加的误区
对用于筛选不同电催化剂催化活性的方法进行批判性研究对于水电解技术的发展至关重要。其中一种证明不同催化剂催化活性趋势的方法是确定其电化学活性表面积(ECSA),该表面积通常根据催化剂材料的非法拉第双层电容(Cdl)估算得出。此外,催化电流与 ECSA 的归一化通常用于深入了解催化剂的内在活性。由于并非所有金属催化剂在电解条件下都具有高度稳定性,因此研究浸出/溶解的多价金属离子的吸附/解吸是否会影响非法拉第双电容的测量非常重要,尽管这种研究很少。 在此,我们探讨了从镍钼合金中浸出的钼对镍钼双电容测量的可能影响。使用循环伏安法(CV)和电化学阻抗谱法(EIS),在碱性条件下进行长期(计时电位计和 CV 循环)氢演化反应(HER)前后测量了镍钼合金的 Cdl。经过长期氢演化反应测试后,我们观察到镍钼合金的 Cdl 在使用传统方法测量时显著上升(所有实验的平均值为 22%)。有趣的是,只有 8% 的升高可归因于表面积的扩大。我们推测,Cdl 增加的大部分原因是浸出的多价离子储存了更多电荷,这些电荷积聚在表面空腔中。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


