Activation of Lattice Oxygen in Nitrogen-Doped High-Entropy Oxide Nanosheets for Highly Efficient Oxygen Evolution Reaction
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
High-entropy oxides (HEOs) are potential electrocatalysts for overcoming the sluggish kinetics of the oxygen evolution reaction (OER). Conventionally, the thermodynamic barrier of the lattice oxygen mechanism (LOM) is lower than that of the adsorbate evolution mechanism (AEM). However, controlling the transition from the AEM to the LOM remains challenging. Herein, an in situ modulation strategy has been developed to synthesize N-FeCoNiAlMoOx by introducing structural directing agents and electronic modulators. Different instruments were used to identify the nitridation-triggered micromorphologies and phase transformations. X-ray photoelectron spectroscopy (XPS) and X-ray absorption fine structure spectroscopy (XAFS) reveal the optimized electronic structures after nitrogen doping. N-FeCoNiAlMox exhibits OER performance with low overpotentials of 240 and 285 mV at 10 and 100 mA·cm–2, respectively. pH dependence, free-radical capture experiments, and density functional theory (DFT) calculations confirm that nitrogen doping facilitates the LOM pathway. This work elucidates nitrogen’s critical role and the LOM pathway’s contribution to efficient OER performance.
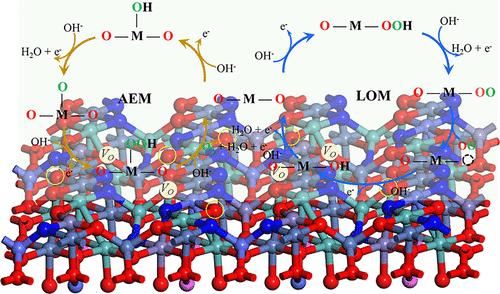
在掺氮高熵氧化物纳米片中激活晶格氧,实现高效氧气进化反应
高熵氧化物(HEOs)是一种潜在的电催化剂,可克服氧进化反应(OER)的动力学迟缓问题。传统上,晶格氧机制(LOM)的热力学势垒低于吸附剂进化机制(AEM)。然而,控制从 AEM 到 LOM 的过渡仍然具有挑战性。在此,我们开发了一种原位调制策略,通过引入结构引导剂和电子调制剂来合成 N-FeCoNiAlMoOx。我们使用了不同的仪器来识别氮化引发的微观形态和相变。X 射线光电子能谱(XPS)和 X 射线吸收精细结构能谱(XAFS)揭示了氮掺杂后优化的电子结构。N-FeCoNiAlMox 在 10 mA-cm-2 和 100 mA-cm-2 条件下分别具有 240 mV 和 285 mV 的低过电位,表现出良好的 OER 性能。pH 依赖性、自由基捕获实验和密度泛函理论(DFT)计算证实,氮掺杂促进了 LOM 途径。这项工作阐明了氮的关键作用以及 LOM 途径对高效 OER 性能的贡献。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


