Sulfur atom occupying surface oxygen vacancy to boost the charge transfer and stability for aqueous Bi2O3 electrode
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
Abstract
Oxygen vacancies (Ov) within metal oxide electrodes can enhance mass/charge transfer dynamics in energy storage systems. However, construction of surface Ov often leads to instability in electrode structure and irreversible electrochemical reactions, posing a significant challenge. To overcome these challenges, atomic heterostructures are employed to address the structural instability and enhance the mass/charge transfer dynamics associated with phase conversion mechanism in aqueous electrodes. Herein, we introduce an atomic S–Bi2O3 heterostructure (sulfur (S) anchoring on the surface Ov of Bi2O3). The integration of S within Bi2O3 lattice matrix triggers a charge imbalance at the heterointerfaces, ultimately resulting in the creation of a built-in electric field (BEF). Thus, the BEF attracts OH− ions to be adsorbed onto Bi within the regions of high electron cloud overlap in S–Bi2O3, facilitating highly efficient charge transfer. Furthermore, the anchored S plays a pivotal role in preserving structural integrity, thus effectively stabilizing the phase conversion reaction of Bi2O3. As a result, the S–Bi2O3 electrode achieves 72.3 mA h g−1 at 10 A g−1 as well as high-capacity retention of 81.9% after 1600 cycles. Our innovative S–Bi2O3 design presents a groundbreaking approach for fabricating electrodes that exhibit efficient and stable mass and charge transfer capabilities. Furthermore, it enhances our understanding of the underlying reaction mechanism within energy storage electrodes.
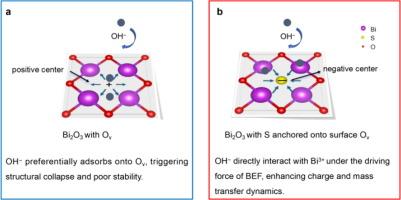
硫原子占据表面氧空位,促进水性 Bi2O3 电极的电荷转移和稳定性
金属氧化物电极中的氧空位(Ov)可增强储能系统中的质量/电荷转移动力学。然而,表面氧空位的形成往往会导致电极结构的不稳定性和不可逆的电化学反应,从而带来巨大的挑战。为了克服这些挑战,我们采用原子异质结构来解决结构不稳定问题,并增强水电极中与相转化机制相关的质量/电荷转移动力学。在此,我们介绍一种原子 S-Bi2O3 异质结构(硫(S)锚定在 Bi2O3 的表面 Ov 上)。S 在 Bi2O3 晶格基质中的整合引发了异质界面的电荷不平衡,最终导致内置电场(BEF)的产生。因此,内置电场吸引 OH 离子在 S-Bi2O3 的高电子云重叠区域内吸附到 Bi 上,从而促进了高效的电荷转移。此外,锚定的 S 在保持结构完整性方面起着关键作用,从而有效地稳定了 Bi2O3 的相转化反应。因此,S-Bi2O3 电极在 10 A g-1 的条件下可达到 72.3 mA h g-1,并在 1600 个循环后实现 81.9% 的高容量保持率。我们创新的 S-Bi2O3 设计为制造具有高效稳定的质量和电荷转移能力的电极提供了一种开创性的方法。此外,它还加深了我们对储能电极内部基本反应机制的理解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


