Tailoring BaCe0.7Zr0.1(Dy0.1|Yb0.1)0.2O3−δ electrolyte through strategic Cu doping for low temperature proton conducting fuel cells: Envisioned theoretically and experimentally
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
Abstract
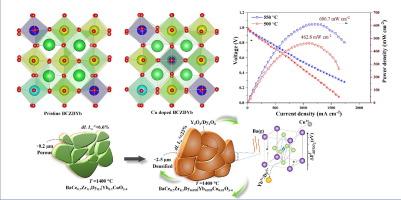
This study addresses the challenge of high sintering temperatures in proton-conducting fuel cells (PCFCs) with BaCeO3-doped electrolytes. We demonstrate that 1 mol% copper (Cu) doping at the B-site of BaCe0.7Zr0.1(Dy0.1|Yb0.1)0.2O3−δ (BCZDYb) improves sintering behavior, enabling densification at 1400 °C. However, Cu doping disrupts stoichiometry, creating barium vacancies and reducing proton-accepting cations, affecting overall conductivity. This mechanism is confirmed through density functional theory (DFT) calculations and various experimental techniques, including crystal structure analysis using X-ray diffraction (XRD) and morphology and elemental analysis via field emission scanning electron microscopy (FESEM) and energy-dispersive X-ray spectroscopy (EDS). Electrochemical measurements are performed using the electrochemical impedance spectroscopy (EIS). The ionic conductivity of 1 mol% Cu-doped BCZDYb (BCZDYb-1) is 1.49 × 10−2 S cm−1 at 650 °C, which is ∼3.58 times higher than that of BCZDYb sintered at 1200 °C. The BCZDYb-1 exhibits ∼16 times higher grain boundary conductivity when sintered at 1400 °C, compared to undoped BCZDYb. The single cell employing BCZDYb-1 as the electrolyte achieved a power density of ∼606 mW cm−2 at 550 °C. These results indicate that a controlled amount of Cu doping can enhance densification while maintaining high ionic conductivity, making it suitable for practical applications in PCFCs operating at lower temperatures.
通过策略性掺铜为低温质子传导燃料电池定制 BaCe0.7Zr0.1(Dy0.1|Yb0.1)0.2O3-δ电解质:理论和实验设想
本研究解决了质子传导燃料电池 (PCFC) 中掺杂 BaCeO3 电解质的高烧结温度难题。我们证明,在 BaCe0.7Zr0.1(Dy0.1|Yb0.1)0.2O3-δ(BCZDYb)的 B 位掺杂 1 摩尔% 的铜(Cu)可改善烧结行为,使其在 1400 ℃ 下实现致密化。然而,掺杂铜会破坏化学计量,产生钡空位并减少质子接受阳离子,从而影响整体导电性。通过密度泛函理论(DFT)计算和各种实验技术,包括利用 X 射线衍射(XRD)进行晶体结构分析,以及通过场发射扫描电子显微镜(FESEM)和能量色散 X 射线光谱(EDS)进行形貌和元素分析,证实了这一机制。电化学测量采用电化学阻抗光谱法(EIS)进行。在 650 °C 时,掺杂 1 mol% Cu 的 BCZDYb(BCZDYb-1)的离子电导率为 1.49 × 10-2 S cm-1,是在 1200 °C 下烧结的 BCZDYb 的 3.58 倍。与未掺杂的 BCZDYb 相比,BCZDYb-1 在 1400 ℃ 烧结时的晶界电导率高出 16 倍。采用 BCZDYb-1 作为电解质的单电池在 550 ℃ 时的功率密度达到了 ∼606 mW cm-2。这些结果表明,可控的铜掺杂量可在保持高离子导电性的同时提高致密性,使其适用于在较低温度下工作的 PCFC 中的实际应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


