Quantification of solvent-mediated host-ion interaction in graphite intercalation compounds for extreme-condition Li-ion batteries
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
Abstract
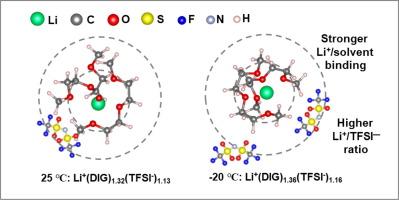
Achieving simultaneous fast-charging capabilities and low-temperature adaptability in graphite-based lithium-ion batteries (LIBs) with an acceptable cycle life remains challenging. Herein, an ether-based electrolyte with temperature-adaptive Li+ solvation structure is designed for graphite, and stable Li+/solvent co-intercalation has been achieved at subzero. As revealed by in-situ variable temperature (−20 °C) X-ray diffraction (XRD), the poor compatibility of graphite in ether-based electrolyte at 25 °C is mainly due to the continuous electrolyte decomposition and the in-plane rearrangement below 0.5 V. Former results in a significant irreversible capacity, while latter maintains graphite in a prolonged state of extreme expansion, ultimately leading to its exfoliation and failure. In contrast, low temperature triggers the rearrangement of Li+ solvation structure with stronger Li+/solvent binding energy and shorter Li+–O bond length, which is conducive for reversible Li+/solvent co-intercalation and reducing the time of graphite in an extreme expansion state. In addition, the co-intercalation of solvents minimizes the interaction between Li-ions and host graphite, endowing graphite with fast diffusion kinetics. As expected, the graphite anode delivers about 84% of the capacity at room temperature at −20 °C. Moreover, within 6 min, about 83%, 73%, and 43% of the capacity could be charged at 25, −20, and −40 °C, respectively.
量化极端条件下锂离子电池石墨插层化合物中溶剂介导的宿主离子相互作用
在石墨基锂离子电池(LIB)中同时实现快速充电能力和低温适应性,并达到可接受的循环寿命,仍然是一项挑战。在此,我们为石墨设计了一种具有温度适应性 Li+ 溶胶结构的醚基电解质,并在零度以下实现了稳定的 Li+ / 溶剂共掺合。原位变温(-20 °C)X 射线衍射(XRD)显示,石墨在 25 °C醚基电解质中的相容性较差,主要是由于电解质在 0.5 V 以下的持续分解和面内重排。前者会导致大量不可逆容量,后者会使石墨长期处于极度膨胀状态,最终导致石墨剥落和失效。相反,低温会引发 Li+ 溶解结构的重新排列,使 Li+ 与溶剂的结合能增强,Li+-O 键长度缩短,有利于 Li+ 与溶剂的可逆共掺杂,缩短石墨处于极度膨胀状态的时间。此外,溶剂的共掺杂将锂离子与寄主石墨之间的相互作用降至最低,使石墨具有快速扩散动力学。正如预期的那样,石墨阳极在-20 °C的室温下可提供约84%的容量。此外,在 25 ℃、-20 ℃ 和 -40 ℃ 下,6 分钟内可分别充入约 83%、73% 和 43% 的容量。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


