The interfacial charge change enhanced by Pr0.6Sm0.4Co0·8Mn0·2O3 activated peroxymonosulfate was used for the efficient degradation of tetracycline under the nanoscale domain limiting and distance effect
IF 7.1
3区 材料科学
Q1 GREEN & SUSTAINABLE SCIENCE & TECHNOLOGY
引用次数: 0
Abstract
In this work, an improved sol-gel method was used to prepare a series of Co-infused PrSmMnO3 perovskites (Pr0.6Sm0.4Co1-XMnXO3, PSCM) to rapidly activate peroxymonosulfate (PMS) for efficient degradation of tetracycline (TC). The experimental results showed that the degradation efficiency of the PSCM-82/PMS system for TC in the pH 2–11 range was close to 100%. The nanoscale domain-limiting and distance effect of PSCM under different activators were discussed, and the catalytic mechanism of the non-radical electron transfer pathway in the PSCM-82/PMS system was proposed. After the optimization of the DFT calculation, it can be seen that the dCo-Mn-Co range of the PSCM-82 material is 9.8–9.9 Å. The molecular size of PMS is 9.9 Å, which precisely matches the dCo-Mn-Co range of the material. Moreover, the distance between molecular sizes was relatively minimal, and the interface charge transfer was enhanced by both the confinement and distance effects. This promotes a fast catalytic reaction and an optimal degradation rate. During this process, PMS molecules were adsorbed by the active metal sites on the surface of PSCM-82, resulting in a large amount of interfacial charge transfer. This allows a strong coupling between the PMS and the catalyst, resulting in a reaction surface with high redox potential. According to the results of density functional theory (DFT) calculation, quenching experiment, electron paramagnetic resonance (EPR) experiment and electrochemical research, it can be concluded that the main degradation pathway of TC is realized through the direct electron transfer process.
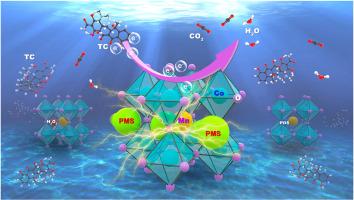
在纳米尺度畴限制和距离效应下,Pr0.6Sm0.4Co0-8Mn0-2O3活化过硫酸盐增强的界面电荷变化被用于高效降解四环素
本研究采用改进的溶胶-凝胶法制备了一系列共注入型 PrSmMnO3 包晶石(Pr0.6Sm0.4Co1-XMnXO3,PSCM),用于快速激活过一硫酸盐(PMS)以高效降解四环素(TC)。实验结果表明,在 pH 值为 2-11 的范围内,PSCM-82/PMS 系统对四环素的降解效率接近 100%。讨论了不同活化剂作用下PSCM的纳米尺度限域效应和距离效应,提出了PSCM-82/PMS体系非自由基电子传递途径的催化机理。经过 DFT 计算优化后可知,PSCM-82 材料的 dCo-Mn-Co 范围为 9.8-9.9 Å,而 PMS 的分子尺寸为 9.9 Å,正好与该材料的 dCo-Mn-Co 范围相匹配。此外,分子尺寸之间的距离相对较小,在约束效应和距离效应的双重作用下,界面电荷转移得到了增强。这促进了快速催化反应和最佳降解率。在此过程中,PMS 分子被 PSCM-82 表面的活性金属位点吸附,从而产生大量的界面电荷转移。这使得 PMS 与催化剂之间产生了强烈的耦合,从而形成了具有高氧化还原电位的反应表面。根据密度泛函理论(DFT)计算、淬火实验、电子顺磁共振(EPR)实验和电化学研究的结果,可以得出 TC 的主要降解途径是通过直接电子转移过程实现的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Sustainability
Multiple-
CiteScore
5.80
自引率
6.40%
发文量
174
审稿时长
32 days
期刊介绍:
Materials Today Sustainability is a multi-disciplinary journal covering all aspects of sustainability through materials science.
With a rapidly increasing population with growing demands, materials science has emerged as a critical discipline toward protecting of the environment and ensuring the long term survival of future generations.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


