Magnetic activated carbonaceous materials from sugarcane bagasse: Preparation, characterization, and hexavalent chromium removal
IF 7.1
3区 材料科学
Q1 GREEN & SUSTAINABLE SCIENCE & TECHNOLOGY
引用次数: 0
Abstract
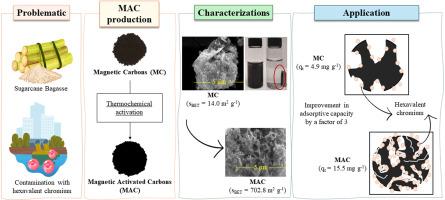
The presence of hexavalent chromium (Cr (VI)) in effluents remains a global concern due to its toxic properties. Even though adsorption has been employed for its removal from water, developing sustainable materials with higher adsorption capacities is still pivotal to tackling such contamination. In this study, two magnetic carbons (MC) were produced by hydrothermal carbonization (HTC) of sugarcane bagasse in the presence of iron (III) nitrate at 230 and 270 °C. Both MCs were thermochemically activated at 500 and 700 °C using KOH (1:2; w:w). The materials were characterized in terms of composition, structure, morphology, texture, and surface properties and then evaluated in adsorption studies of Cr (VI). After HTC, some iron phases such as α-Fe2O3, γ-Fe2O3, and Fe3O4 were observed, while thermochemical activation additionally revealed Fe0 and Fe4[Fe(CN)6]3. Activation increased the amount of meso- and macropores, specific surface area, pHzpc, surface hydrophilicity, and carbon and nitrogen contents. The adsorption kinetics study indicated that the pseudo-second-order model describes better the behavior of the materials. The investigation of adsorbent dose showed that doses below 1.00 g L−1 were more efficient in Cr (VI) removal. MC-230 and MC-270 thermochemically activated at 700 °C exhibited the highest Cr (VI) adsorption capacities (10.5 and 15.5 mg g−1, respectively). Therefore, the improved adsorption capacity for Cr (VI) of the materials thermochemically activated at 700 °C was mainly due to their enhanced textural properties.
甘蔗渣磁性活性炭材料:制备、表征和六价铬去除
由于六价铬(Cr (VI))的毒性,其在污水中的存在仍然是一个全球关注的问题。尽管吸附技术已被用于去除水中的六价铬,但开发吸附能力更强的可持续材料仍是解决此类污染的关键。在本研究中,甘蔗渣在硝酸铁(III)存在下于 230 和 270 °C 下通过水热碳化(HTC)制备了两种磁性碳(MC)。两种 MC 均在 500 和 700 °C 下使用 KOH(1:2;w:w)进行热化学活化。对材料的组成、结构、形态、质地和表面特性进行了表征,然后在 Cr (VI) 吸附研究中进行了评估。在 HTC 之后,观察到一些铁相,如 α-Fe2O3、γ-Fe2O3 和 Fe3O4,而热化学活化还发现了 Fe0 和 Fe4[Fe(CN)6]3。活化增加了中孔和大孔的数量、比表面积、pHzpc、表面亲水性以及碳和氮的含量。吸附动力学研究表明,伪二阶模型能更好地描述材料的行为。对吸附剂剂量的研究表明,低于 1.00 g L-1 的剂量对六价铬的去除更有效。在 700 °C 下热化学活化的 MC-230 和 MC-270 对六价铬的吸附容量最高(分别为 10.5 和 15.5 mg g-1)。因此,在 700 ℃ 下热化学活化的材料对六价铬的吸附能力提高主要是由于它们的质构特性增强了。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Sustainability
Multiple-
CiteScore
5.80
自引率
6.40%
发文量
174
审稿时长
32 days
期刊介绍:
Materials Today Sustainability is a multi-disciplinary journal covering all aspects of sustainability through materials science.
With a rapidly increasing population with growing demands, materials science has emerged as a critical discipline toward protecting of the environment and ensuring the long term survival of future generations.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


