Comparative study of zirconium-zinc oxide thin films on ceramic and glass substrates: Structural, optical, and photocatalytic properties
IF 4.4
3区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
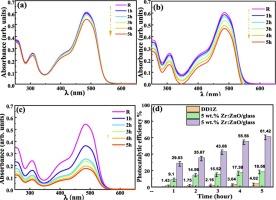
This study investigates the structural, optical, and photocatalytic properties of zirconium-doped zinc oxide (Zr) thin films deposited on ceramic and glass substrates. X-ray diffraction (XRD) analysis revealed that all samples exhibited a polycrystalline wurtzite structure, with grain sizes ranging from 25 to 43 nm for ceramic substrates and 19 to 32 nm for glass substrates. UV–visible absorbance measurements indicated that Zr films absorbed visible light around 410 nm, with the band gap widening to 2.93 eV on ceramic substrates. Scanning electron microscopy (SEM) showed that Zr doping significantly affected the morphology of thin films, resulting in rougher surfaces and larger grains on ceramic substrates. Photocatalytic tests demonstrated that the 5 wt% Zr on DD1Z ceramic achieved a degradation efficiency of 61.44 % for orange II dye after 5 h of UV irradiation. In contrast, Zr on ceramic substrates achieved only 18.56 %, and the bare DD1Z substrate reached just 4.02 %. These findings highlight the superior photocatalytic performance of Zr on ceramic substrates, indicating their potential for advanced water purification technologies. The mechanism of photolysis was investigated using hole/radical scavengers, revealing that Zr-ZnO networks enhance the adsorption of hydroxyl ions on the surface. This acts as a trap site, reducing hole/electron pair recombination and thus increasing activity and photodegradation efficiency.
陶瓷和玻璃基底上氧化锆-氧化锌薄膜的比较研究:结构、光学和光催化特性
本研究探讨了沉积在陶瓷和玻璃基底上的掺锆氧化锌(Zr)薄膜的结构、光学和光催化特性。X 射线衍射(XRD)分析表明,所有样品都呈现出多晶钨酸盐结构,陶瓷基底的晶粒大小在 25 至 43 纳米之间,玻璃基底的晶粒大小在 19 至 32 纳米之间。紫外-可见吸收率测量结果表明,Zr 薄膜吸收 410 纳米波长左右的可见光,陶瓷基底的带隙扩大到 2.93 eV。扫描电子显微镜(SEM)显示,掺杂 Zr 显著影响了薄膜的形态,导致陶瓷基底上的薄膜表面更粗糙,晶粒更大。光催化测试表明,在紫外线照射 5 小时后,DD1Z 陶瓷上 5 wt% 的 Zr 对橙 II 染料的降解效率达到 61.44%。相比之下,陶瓷基底上的 Zr 降解效率仅为 18.56%,而裸露的 DD1Z 基底仅为 4.02%。这些发现凸显了陶瓷基底上的 Zr 具有卓越的光催化性能,表明其具有应用于先进水净化技术的潜力。利用空穴/自由基清除剂对光解机制进行了研究,发现 Zr-ZnO 网络增强了表面对羟基离子的吸附。这就起到了捕集作用,减少了空穴/电子对重组,从而提高了活性和光降解效率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry Communications
化学-无机化学与核化学
CiteScore
5.50
自引率
7.90%
发文量
1013
审稿时长
53 days
期刊介绍:
Launched in January 1998, Inorganic Chemistry Communications is an international journal dedicated to the rapid publication of short communications in the major areas of inorganic, organometallic and supramolecular chemistry. Topics include synthetic and reaction chemistry, kinetics and mechanisms of reactions, bioinorganic chemistry, photochemistry and the use of metal and organometallic compounds in stoichiometric and catalytic synthesis or organic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


