A novel azo-triazolopyridine-based dual naked-eye chemosensor for the selective detection of CN− and Cu2+ ions
IF 4.1
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2024-11-07
DOI:10.1016/j.jphotochem.2024.116140
引用次数: 0
Abstract
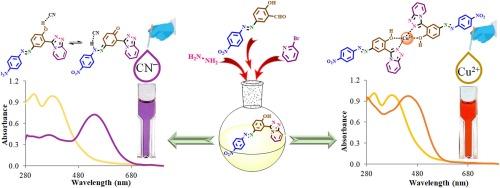
A new chemosensor incorporating a triazolopyridine and an azo chromophore was synthesized and employed as a colorimetric sensor. Visual investigations for detecting anions and cations revealed that the sensor acts as a highly sensitive and selective chromogenic detector for cyanide (CN−) and copper (Cu2+) ions. The observable color change from yellow to deep orange for Cu2+ and from yellow to purple for CN− allows for detecting these ions without the need for advanced equipment, even with the naked eye. The sensor’s limit of detection (LOD) was determined to be 0.02 μM for CN− ions and 1.13 μM for Cu2+ ions. The Job plot indicated that the sensor binds to CN− and Cu2+ with a stoichiometric ratio of 1:1 and 2:1, respectively. The ability to accurately and selectively distinguish Cu2+ and CN− ions makes this sensor valuable for various analytical applications, including environmental monitoring and chemical analysis. Its capacity to produce noticeable and distinct color changes enables the rapid and straightforward detection of these hazardous ions, making it a practical choice for real-world applications. The quantum yield of A-TAP/Cu2+ was 54 % at a λmax (maximum absorbance) of 450 nm, while that of A-TAP/CN− was 62 % at 538 nm, using a reference sensor with a λmax of 380 nm.
基于偶氮-三唑并吡啶的新型裸眼双化学传感器,用于选择性检测 CN- 和 Cu2+ 离子
我们合成了一种包含三唑并吡啶和偶氮发色团的新型化学传感器,并将其用作比色传感器。检测阴离子和阳离子的目测结果表明,该传感器对氰化物(CN-)和铜离子(Cu2+)具有高灵敏度和选择性。Cu2+ 的颜色从黄色变为深橙色,CN- 的颜色从黄色变为紫色,这种可观察到的颜色变化无需先进设备,甚至用肉眼就能检测到这些离子。传感器的检测限(LOD)被确定为 CN- 离子 0.02 μM,Cu2+ 离子 1.13 μM。约伯图表明,传感器与 CN- 和 Cu2+ 的结合比例分别为 1:1 和 2:1。这种传感器能够准确并有选择性地区分 Cu2+ 和 CN- 离子,因此在环境监测和化学分析等各种分析应用中具有重要价值。它能够产生明显而独特的颜色变化,从而能够快速、直接地检测这些有害离子,使其成为实际应用中的一个实用选择。使用 λmax 为 380 nm 的参考传感器,A-TAP/Cu2+ 在 450 nm λmax(最大吸光度)处的量子产率为 54%,而 A-TAP/CN- 在 538 nm 处的量子产率为 62%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


