Boosted denitration activity of α-Fe2O3 for low-temperature NH3-SCR by addition of Ce/Cu
IF 5.4
3区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
To enhance the low-temperature denitration efficiency of α-Fe2O3, Ce/Cu-supported Ce/α-Fe and Cu/α-Fe were synthesized and applied to NH3-SCR denitration. The findings indicate that Ce and Cu significantly improve the low-temperature denitration performance of α-Fe2O3, with the Cu/α-Fe achieving over 95 % catalytic efficiency between 240 and 360 °C. Characterization results show strong interactions and electronic transfer effects between Ce/Cu and Fe, which significantly reduce the crystallinity of α-Fe2O3 and generate more surface Fe3+ and chemisorbed oxygen. For Cu/α-Fe, its low-temperature denitration performance is largely dependent on its redox capacity and surface acidity. In contrast, the improved low-temperature denitration performance of Ce/α-Fe is mainly attributed to its significantly enhanced surface acidity. Moreover, after loading Ce and Cu, the types of surface adsorbed NOx species are increased. Particularly for Cu/α-Fe, monodentate nitrate is newly generated, which are key active species for low-temperature catalytic activity, explaining why Cu/α-Fe exhibits the best low-temperature catalytic performance.
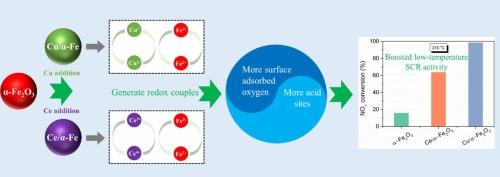
通过添加 Ce/Cu 提高用于低温 NH3-SCR 的 α-Fe2O3 的脱硝活性
为了提高α-Fe2O3的低温脱硝效率,合成了Ce/Cu支撑的Ce/α-Fe和Cu/α-Fe,并将其应用于NH3-SCR脱硝。研究结果表明,Ce 和 Cu 能显著改善 α-Fe2O3 的低温脱硝性能,Cu/α-Fe 在 240 至 360 °C 之间的催化效率超过 95%。表征结果表明,Ce/Cu 和 Fe 之间存在很强的相互作用和电子转移效应,这大大降低了 α-Fe2O3 的结晶度,并产生了更多的表面 Fe3+ 和化学吸附氧。对于 Cu/α-Fe 来说,其低温脱硝性能主要取决于其氧化还原能力和表面酸度。相比之下,Ce/α-Fe 的低温脱硝性能之所以得到改善,主要是因为其表面酸度显著增强。此外,添加 Ce 和 Cu 后,表面吸附的氮氧化物种类也有所增加。特别是对于 Cu/α-Fe 来说,新生成的单齿硝酸盐是低温催化活性的关键活性物种,这也解释了为什么 Cu/α-Fe 具有最佳的低温催化性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry Communications
化学-无机化学与核化学
CiteScore
5.50
自引率
7.90%
发文量
1013
审稿时长
53 days
期刊介绍:
Launched in January 1998, Inorganic Chemistry Communications is an international journal dedicated to the rapid publication of short communications in the major areas of inorganic, organometallic and supramolecular chemistry. Topics include synthetic and reaction chemistry, kinetics and mechanisms of reactions, bioinorganic chemistry, photochemistry and the use of metal and organometallic compounds in stoichiometric and catalytic synthesis or organic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


