The BNT162b2 mRNA vaccine demonstrates reduced age-associated TH1 support in vitro and in vivo
IF 4.6
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
mRNA vaccines demonstrate impaired immunogenicity and durability in vulnerable older populations. We hypothesized that human in vitro modeling and proteomics could elucidate age-specific mRNA vaccine actions. BNT162b2-stimulation changed the plasma proteome of blood samples from young (18-50Y) and older adult (≥60Y) participants, assessed by mass spectrometry, proximity extension assay, and multiplex. Young adult up-regulation (e.g., PSMC6, CPN1) contrasted reduced induction in older adults (e.g., TPM4, APOF, APOC2, CPN1, PI16). 30–85% lower TH1-polarizing cytokines and chemokines were induced in elderly blood (e.g., IFNγ, CXCL10). Analytes lower in older adult samples included human in vivo mRNA immunogenicity biomarkers (e.g., IFNγ, CXCL10, CCL4, IL-1RA). BNT162b2 also demonstrated reduced CD4+ TH1 responses in aged vs. young adult mice. Our study demonstrates the utility of human in vitro platforms modeling age-specific mRNA vaccine immunogenicity, highlights impaired support of TH1 polarization in older adults, and provides a rationale for precision mRNA vaccine adjuvantation to induce greater immunogenicity.
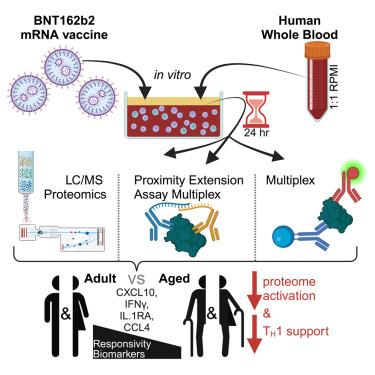
BNT162b2 mRNA 疫苗在体外和体内都能降低年龄相关的 TH1 支持率
mRNA 疫苗在易受影响的老年人群中显示出免疫原性和持久性受损。我们假设人体体外建模和蛋白质组学可以阐明特定年龄的 mRNA 疫苗作用。BNT162b2 刺激改变了年轻人(18-50 岁)和老年人(≥60 岁)血液样本的血浆蛋白质组,并通过质谱分析、近距离延伸测定和多重分析进行了评估。年轻人的上调(如 PSMC6、CPN1)与老年人的诱导减少(如 TPM4、APOF、APOC2、CPN1、PI16)形成鲜明对比。老年人血液中诱导的 TH1 极化细胞因子和趋化因子(如 IFNγ、CXCL10)降低了 30-85%。老年人样本中含量较低的分析物包括人体内 mRNA 免疫原性生物标记物(如 IFNγ、CXCL10、CCL4、IL-1RA)。BNT162b2 还显示,老年小鼠与年轻成年小鼠的 CD4+ TH1 反应减少。我们的研究证明了人类体外平台模拟年龄特异性 mRNA 疫苗免疫原性的实用性,强调了老年人对 TH1 极化的支持受损,并为精确 mRNA 疫苗佐剂诱导更大的免疫原性提供了理论依据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


