Reaction between peracetic acid and carbonyl oxide: Quantitative kinetics and insight into implications in the atmosphere
IF 4.2
2区 环境科学与生态学
Q2 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
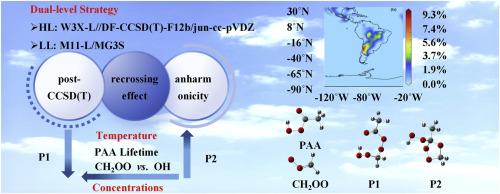
Peracetic acid (PAA, CH3C(O)OOH) is one of the most abundant organic peroxyacid in the atmosphere. PAA is often assumed to be removed by hydroxyl radical in the gas phase of troposphere, but its reaction rate is quite low. Here, we investigated the new reaction between PAA and carbonyl oxide (CH2OO) by using quantum chemical methods, reaction kinetics in combination with atmospheric modeling. We first performed W3X-L calculations close to CCSDT(Q)/CBS accuracy with the reaction systems containing eight carbon and oxygen atoms. The present findings show that the post-CCSD(T) contribution is about 0.50 kcal/mol, which is important for obtaining quantitative relative enthalpy of activation at 0 K. We find that the recrossing effect reduces the rate constant by an order of magnitude for the mechanism of the hydrogen-shift coupled carbon-oxygen addition at low temperature. The calculated results reveal that the anharmonicity increases the rate constants of CH2OO + CH3C(O)OOH by a factor of 6.27 at 298 K. The present findings uncover that the PAA + CH2OO reaction is a dominant pathway for PAA sinks in the gas phase of troposphere at the lower nighttime OH concentrations at 298 K, since the rate of PAA + CH2OO is even an order of magnitude higher than the rate of the PAA + OH reaction. Moreover, atmospheric modeling simulations unveil that CH2OO can make certain contribution to the reduction of PAA in the Amazon.
过乙酸与羰基氧化物之间的反应:定量动力学和对大气中影响的见解
过乙酸(PAA,CH3C(O)OOH)是大气中含量最高的有机过氧酸之一。人们通常认为 PAA 在对流层气相中会被羟基自由基清除,但其反应速率相当低。在此,我们采用量子化学方法、反应动力学和大气模型相结合的方法研究了 PAA 和羰基氧化物(CH2OO)之间的新反应。我们首先对含有八个碳原子和氧原子的反应体系进行了接近 CCSDT(Q)/CBS 精确度的 W3X-L 计算。本研究结果表明,CCSD(T) 后贡献约为 0.50 kcal/mol,这对于获得 0 K 时的定量相对活化焓非常重要。我们发现,对于低温下氢移耦合碳氧加成的机理而言,交叉效应将速率常数降低了一个数量级。计算结果显示,在 298 K 时,非谐波使 CH2OO + CH3C(O)OOH 的速率常数增加了 6.27 倍。本研究结果发现,在 298 K 的较低夜间 OH 浓度条件下,PAA + CH2OO 反应是对流层气相中 PAA 吸收的主要途径,因为 PAA + CH2OO 的速率甚至比 PAA + OH 反应的速率高出一个数量级。此外,大气模型模拟显示,CH2OO 对亚马逊河流域 PAA 的减少有一定的贡献。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Atmospheric Environment
环境科学-环境科学
CiteScore
9.40
自引率
8.00%
发文量
458
审稿时长
53 days
期刊介绍:
Atmospheric Environment has an open access mirror journal Atmospheric Environment: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Atmospheric Environment is the international journal for scientists in different disciplines related to atmospheric composition and its impacts. The journal publishes scientific articles with atmospheric relevance of emissions and depositions of gaseous and particulate compounds, chemical processes and physical effects in the atmosphere, as well as impacts of the changing atmospheric composition on human health, air quality, climate change, and ecosystems.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


