Disparity between rate and selectivity in the controlled synthesis of gradient conjugated copolymers
IF 5.8
2区 化学
Q1 POLYMER SCIENCE
引用次数: 0
Abstract
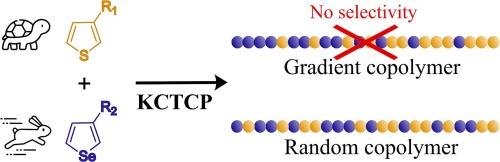
In this report, a one-pot synthesis of gradient copolymers is attempted employing Kumada catalyst transfer condensative polymerization (KCTCP). The hypothesis that a more reactive monomer is preferentially being built into the chain at the beginning, while the slower reacting monomer is only being built into the chain at the end due to it being in excess, is investigated. The monomer rate constants are influenced by the usage of differently sized sidechains (octyl versus 3-octyldodecyl) and nucleophilicity of the monomers (thiophene versus selenophene). Nonetheless, while the rate constants differ by a factor 2, no selectivity is obtained. The cause thereof is further investigated by increasing the sterical crowdedness around the catalyst-complex by employing a 4-substituted monomer, the polymerization of which also gave rise to random copolymers. Accompanied by density functional theory calculations of the geometry and Gibbs free energy, it is concluded that the distance between the nickel catalyst and the polymer chain is much smaller compared to the distance between the catalyst and the incoming monomer. This results in different polymerization rates (depending on the polymer-nickel complex), while having no selectivity for one incoming monomer over the other.
梯度共轭共聚物受控合成中速率与选择性之间的差异
本报告尝试采用 Kumada 催化剂转移冷凝聚合法 (KCTCP) 单锅合成梯度共聚物。我们研究了这样一种假设,即反应性较强的单体在开始时优先被加入链中,而反应性较弱的单体由于过量而只在末端被加入链中。单体速率常数受到不同大小侧链(辛基与 3-辛基十二烷基)的使用和单体亲核性(噻吩与硒吩)的影响。然而,虽然速率常数相差 2 倍,但却没有获得选择性。通过使用 4 取代单体增加催化剂复合物周围的立体拥挤度,进一步研究了其原因。通过对几何形状和吉布斯自由能的密度泛函理论计算得出结论,镍催化剂与聚合物链之间的距离比催化剂与进入的单体之间的距离要小得多。这导致了不同的聚合速率(取决于聚合物-镍复合物),同时对一种进入的单体没有选择性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

European Polymer Journal
化学-高分子科学
CiteScore
9.90
自引率
10.00%
发文量
691
审稿时长
23 days
期刊介绍:
European Polymer Journal is dedicated to publishing work on fundamental and applied polymer chemistry and macromolecular materials. The journal covers all aspects of polymer synthesis, including polymerization mechanisms and chemical functional transformations, with a focus on novel polymers and the relationships between molecular structure and polymer properties. In addition, we welcome submissions on bio-based or renewable polymers, stimuli-responsive systems and polymer bio-hybrids. European Polymer Journal also publishes research on the biomedical application of polymers, including drug delivery and regenerative medicine. The main scope is covered but not limited to the following core research areas:
Polymer synthesis and functionalization
• Novel synthetic routes for polymerization, functional modification, controlled/living polymerization and precision polymers.
Stimuli-responsive polymers
• Including shape memory and self-healing polymers.
Supramolecular polymers and self-assembly
• Molecular recognition and higher order polymer structures.
Renewable and sustainable polymers
• Bio-based, biodegradable and anti-microbial polymers and polymeric bio-nanocomposites.
Polymers at interfaces and surfaces
• Chemistry and engineering of surfaces with biological relevance, including patterning, antifouling polymers and polymers for membrane applications.
Biomedical applications and nanomedicine
• Polymers for regenerative medicine, drug delivery molecular release and gene therapy
The scope of European Polymer Journal no longer includes Polymer Physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


