Synthesis and evaluation of cyclic peptide-dasatinib conjugates as anti-melanoma agents
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
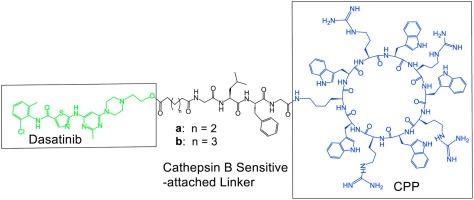
Herein, we synthesized two amphiphilic cyclic peptides, incorporating tryptophan and arginine residues, linked to dasatinib via a Cathepsin B-sensitive tetrapeptide (Gly-Phe-Leu-Gly). To mitigate steric hindrance and optimize drug release, we integrated two different linkers, succinate and glutarate, between the drug and peptide. The synthesis involved solid-phase techniques for the assembly of the peptide, followed by the coupling of the peptide on-resin with the linker, mild cleavage, and solution-phase cyclization. Conjugation of the peptide-attached linker with dasatinib was conducted in the presence of N-methylmorpholine and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), generating the novel prodrugs. The synthesized compounds were characterized by high-resolution mass spectrometry MALDI, and the purity was confirmed by analytical HPLC. The synthesized cyclic peptide-dasatinib conjugates containing glutarate and succinate linkers were further evaluated against human melanoma A375 cells, which exhibited IC50 values of 4.2 μM and 8.8 μM, respectively. Variations in cytotoxicity could be attributed to linker properties affecting intracellular drug release or prodrug uptake.
作为抗黑色素瘤药物的环肽-达沙替尼共轭物的合成与评估
在这里,我们合成了两种两亲性环肽,其中包含色氨酸和精氨酸残基,并通过对胰蛋白酶 B 敏感的四肽(Gly-Phe-Leu-Gly)与达沙替尼连接。为了减轻立体阻碍和优化药物释放,我们在药物和肽之间加入了两种不同的连接物--琥珀酸和戊二酸。合成过程采用固相技术组装多肽,然后将多肽在树脂上与连接体偶联,再进行温和的裂解和溶液相环化。在 N-甲基吗啉和 1-乙基-3-(3-二甲胺基丙基)碳二亚胺(EDC)存在下,将肽连接的连接体与达沙替尼共轭,生成新型原药。合成的化合物经高分辨率 MALDI 质谱鉴定,纯度经分析 HPLC 确认。合成的含有戊二酸和琥珀酸连接体的环肽-达沙替尼共轭物对人类黑色素瘤 A375 细胞进行了进一步评估,其 IC50 值分别为 4.2 μM 和 8.8 μM。细胞毒性的变化可能是由于连接体的特性影响了细胞内的药物释放或原药吸收。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


