Diastereoselective synthesis of novel alkaloid-like 1,2,3,3a,7b,12-hexahydroindeno[2′,1′:2,3]indeno[1,2-c]pyrroles
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
2-Arylideneindan-1,3-diones readily undergo [3+2]-cycloaddition with N-methylazomethine ylide derived from sarcosine and formaldehyde to give 4′-aryl-1′-methylspiro[indene-2,3′-pyrrolidine]-1,3-diones, which were further cyclized in perchloric acid to (3aR*,7bR*,12aR*)-7b-hydroxy-2-methyl-2,3,3a,7b-tetrahydroindeno[2′,1′:2,3]indeno[1,2-c]pyrrol-12(1H)-ones in 33–100 % yields.
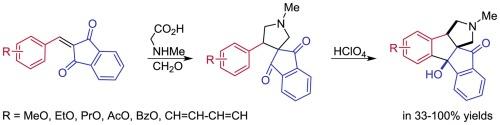
新型生物碱样 1,2,3,3a,7b,12-六氢茚并[2′,1′:2,3]茚并[1,2-c]吡咯的非对映选择性合成
2-Arylideneindan-1,3-diones 很容易与从肌氨酸和甲醛中提取的 N-甲基氮杂环丁烷发生 [3+2]-cycloaddition 反应,生成 4′-芳基-1′-甲基螺[茚-2、3′-吡咯烷]-1,3-二酮,在高氯酸中进一步环化为(3aR*,7bR*,12aR*)-7b-羟基-2-甲基-2,3,3a,7b-四氢茚并[2′,1′:2,3]茚并[1,2-c]吡咯-12(1H)-酮,收率为 33-100%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


