Synthesis of zirconium-based metal-organic framework under mild conditions and its application to the removal of cationic and anionic dyes from wastewater
IF 4.3
3区 材料科学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
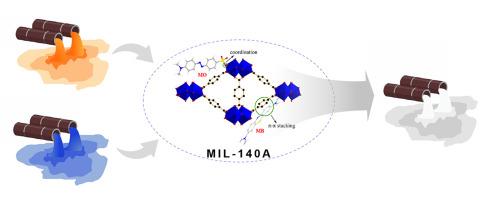
Water resources contaminated by industrial dyes can pose a significant threat to the environment and human health. Herein, we conducted a study on the removal of cationic and anionic dyes, such as methylene blue (MB) and methyl orange (MO), using MIL-140A, a zirconium-based metal-organic framework. MIL-140A is synthesized in a Schlenk flask at 120 °C, whereas its conventional synthesis route involves a teflon-sealed autoclave at 220 °C, highlighting the cost reduction and lower equipment requirements of the low-temperature synthesis. The structure of MIL-140A is characterized by X-ray diffraction (XRD), thermogravimetric analysis (TGA), scanning electron microscope (SEM), and nitrogen adsorption techniques. The optimal pH for the adsorption of two dyes by MIL-140A is pH 5–8 for MB and pH 3 for MO. The adsorption equilibrium can be reached within 60 min at room temperature, and the adsorption of both dyes on MIL-140A follows pseudo-second-order kinetics and Langmuir isotherm, and the maximum adsorption capacity of MO and MB by MIL-140A were 163.6 and 89.2 mg/g, respectively. Thermodynamic studies indicate an entropy-driven spontaneous process. The adsorption mechanism of MO and MB on MIL-140A is investigated using FT-IR and X-ray photoelectron spectroscopy. The adsorption of MO involves coordination between Zr and sulfonate, while MB adsorption occurs via π-π interactions. Additionally, MIL-140A exhibits better removal efficiency for MO from lithium battery wastewater compared to MB, primarily due to stronger coordination interactions than π-π interactions. These findings demonstrate that MIL-140A is a promising adsorbent for effectively removing both anionic and cationic dyes from water resources.
在温和条件下合成锆基金属有机框架并将其应用于去除废水中的阳离子和阴离子染料
受工业染料污染的水资源会对环境和人类健康造成严重威胁。在此,我们利用锆基金属有机框架 MIL-140A 进行了一项关于去除亚甲基蓝(MB)和甲基橙(MO)等阳离子和阴离子染料的研究。MIL-140A 是在 120 ℃ 的施伦克烧瓶中合成的,而其传统合成路线需要在 220 ℃ 的聚四氟乙烯密封高压锅中进行,这突出表明低温合成可降低成本和设备要求。通过 X 射线衍射 (XRD)、热重分析 (TGA)、扫描电子显微镜 (SEM) 和氮吸附技术对 MIL-140A 的结构进行了表征。MIL-140A 吸附两种染料的最佳 pH 值为:MB 为 pH 5-8,MO 为 pH 3。两种染料在 MIL-140A 上的吸附遵循伪二阶动力学和 Langmuir 等温线,MO 和 MB 在 MIL-140A 上的最大吸附容量分别为 163.6 和 89.2 mg/g。热力学研究表明这是一个熵驱动的自发过程。利用傅立叶变换红外光谱和 X 射线光电子能谱研究了 MO 和 MB 在 MIL-140A 上的吸附机理。MO 的吸附涉及 Zr 与磺酸盐之间的配位,而 MB 则通过 π-π 相互作用吸附。此外,与 MB 相比,MIL-140A 对锂电池废水中 MO 的去除效率更高,这主要是由于配位作用比 π-π 作用更强。这些研究结果表明,MIL-140A 是一种很有前途的吸附剂,可有效去除水资源中的阴离子和阳离子染料。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
2.50%
发文量
605
审稿时长
40 days
期刊介绍:
The Journal of Physics and Chemistry of Solids is a well-established international medium for publication of archival research in condensed matter and materials sciences. Areas of interest broadly include experimental and theoretical research on electronic, magnetic, spectroscopic and structural properties as well as the statistical mechanics and thermodynamics of materials. The focus is on gaining physical and chemical insight into the properties and potential applications of condensed matter systems.
Within the broad scope of the journal, beyond regular contributions, the editors have identified submissions in the following areas of physics and chemistry of solids to be of special current interest to the journal:
Low-dimensional systems
Exotic states of quantum electron matter including topological phases
Energy conversion and storage
Interfaces, nanoparticles and catalysts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


