Engineered surface design of recognition site-ordered biomimetic sensor for efficient detection of circulating tumor cells
IF 10.7
1区 生物学
Q1 BIOPHYSICS
引用次数: 0
Abstract
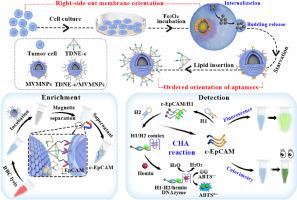
The oriented assembly of cell membrane coating plays an important role in advancing the application of this strategy in biomedical fields, particularly in detecting circulating tumor cells (CTCs). Unfortunately, there is a formidable challenge in achieving effective membrane orientation during the coating process owing to the asymmetric properties of cell membranes. Herein, magnetic vesicles released by tumor cells were designed to break down these barriers in the same way that microvesicles are actively secreted from cells, which completely inherited the orientation and characteristics of the parent cell membranes, exhibiting a satisfactory self-targeting ability for homologous cells. To cope with the complex application environment, spatially ordered aptamers were integrated into magnetic vesicles and combined with catalytic hairpin assembly (CHA) technology to construct a recognition site-ordered biomimetic sensor for high-performance detection of CTCs. In this strategy, the ordered arrangement of membrane proteins and aptamers markedly improved capture efficiency of traditional biomimetic strategy for CTCs. Additionally, CHA-induced fluorescence and colorimetric analysis ensured the detection accuracy and sensitivity, with a linear range of 0 to 104 cells mL−1 and a low detection limit of 3 cells mL−1 for fluorometry and 6 cells mL−1 for colorimetry. Overall, the biomimetic sensor offered broader possibilities for detecting rare CTCs and provided new insight to expand the application of cell membrane biomimetic strategies in biomedicine.
用于高效检测循环肿瘤细胞的识别位点有序生物仿生传感器的工程表面设计
细胞膜包衣的定向组装在推动这一策略在生物医学领域的应用方面发挥着重要作用,尤其是在检测循环肿瘤细胞(CTC)方面。遗憾的是,由于细胞膜的不对称特性,在包膜过程中实现有效的膜定向是一项艰巨的挑战。在此,我们设计了肿瘤细胞释放的磁性囊泡,以打破这些障碍,就像细胞主动分泌微囊泡一样,完全继承了母细胞膜的方向和特性,对同源细胞表现出令人满意的自靶向能力。为了应对复杂的应用环境,我们将空间有序的适配体整合到磁性囊泡中,并结合催化发夹组装(CHA)技术,构建了一种识别位点有序的生物仿生传感器,用于对四氯化碳进行高性能检测。在这一策略中,膜蛋白和适配体的有序排列明显提高了传统生物仿生策略对 CTCs 的捕获效率。此外,CHA 诱导的荧光和比色分析确保了检测的准确性和灵敏度,其线性范围为 0 至 104 cells mL-1,荧光测定的检测限低至 3 cells mL-1,比色测定的检测限低至 6 cells mL-1。总之,生物仿生传感器为检测罕见的四氯化碳提供了更广泛的可能性,并为拓展细胞膜生物仿生策略在生物医学中的应用提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biosensors and Bioelectronics
工程技术-电化学
CiteScore
20.80
自引率
7.10%
发文量
1006
审稿时长
29 days
期刊介绍:
Biosensors & Bioelectronics, along with its open access companion journal Biosensors & Bioelectronics: X, is the leading international publication in the field of biosensors and bioelectronics. It covers research, design, development, and application of biosensors, which are analytical devices incorporating biological materials with physicochemical transducers. These devices, including sensors, DNA chips, electronic noses, and lab-on-a-chip, produce digital signals proportional to specific analytes. Examples include immunosensors and enzyme-based biosensors, applied in various fields such as medicine, environmental monitoring, and food industry. The journal also focuses on molecular and supramolecular structures for enhancing device performance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


