Synthesis of prodelphinidin B2 3,3′′-digallate using AgOTf-mediated self-condensation
IF 2.5
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The Lewis acid mediated self-condensation of an epigallocatechin gallate derivative was examined. We found that AgOTf as a Lewis acid and diethylene glycol as a leaving group at C-4 position afforded a dimeric epigallocatechin gallate derivative in a moderate yield. The condensed product was applied to the synthesis of prodelphinidin B2 3,3′′-digallate.
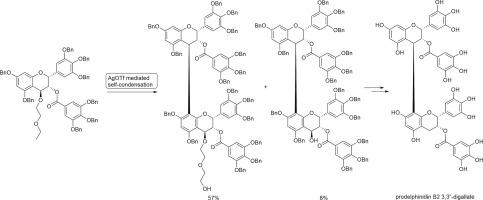
利用 AgOTf 介导的自缩合合成前花翠素 B2 3,3′′-digallate
我们研究了路易斯酸介导的表没食子儿茶素没食子酸酯衍生物的自缩合。我们发现,以 AgOTf 为路易斯酸,二甘醇为 C-4 位上的离去基团,可以得到二聚表没食子儿茶素没食子酸酯衍生物,收率适中。该缩合产物被用于合成前花翠素 B2 3,3′′-二没食子酸酯。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


