Understanding the role of sodium lignosulphonate on obstructing the aggregation of fine serpentine particles on to the hydrophobized pyrite surface
IF 5.7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
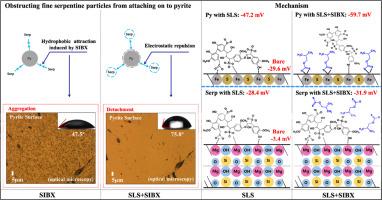
The slime coatings of serpentine via its hydrophobic Si-O layers significantly weaken the floatability of the hydrophobized sulfide minerals. In this paper, sodium lignosulphonate (SLS) was adopted to avoid the aggregation of fine serpentine particles on to the hydrophobized pyrite surface. The micro-flotation findings indicated that for the artificial mixture of pyrite and serpentine (2:1 mass ratio), 1.0 × 10–4 mol/L sodium isobutyl xanthate (SIBX) only floated out ∼22 % pyrite at pH ∼9.0, while, after extra addition of 70 mg/L SLS, the floatability of pyrite was restored and its recovery reached ∼90 %. In situ AFM (Atomic Force Microscope), contact angle, zeta potential, fourier transform infrared spectrometer (FTIR) and optical microscope deduced that the strong affinity of SIBX to pyrite rendered it replace the most part of the pre-aggregated SLS from pyrite surface, thus to restore the hydrophobicity of pyrite and further decrease the zeta potential. In addition, SLS attached on the positively-charged Mg sites of serpentine through its carboxyl and phenolic hydroxyl groups reduce the hydrophobicity and zeta potential of serpentine. While, the hard base O atom/anion exhibited much stronger affinity to hard acid Mg cation than that of the soft base S atom/anion, SIBX hardly replaced the adsorbed SLS from serpentine surface. Therefore, the increased electrostatic repulsion and weakened hydrophobic attraction between the SLS-adsorbed serpentine and SIBX-hydrophobized pyrite avoided their aggregation. The combination of SLS and SIBX realized the selective flotation separation of pyrite from serpentine.
了解木质素磺酸钠在阻碍细小蛇纹石颗粒聚集到疏水黄铁矿表面上的作用
蛇纹石通过其疏水性 Si-O 层形成的粘液涂层大大削弱了疏水性硫化矿物的可浮性。本文采用木质素磺酸钠(SLS)来避免细小蛇纹石颗粒聚集到疏水性黄铁矿表面。微浮选结果表明,对于黄铁矿和蛇纹石的人工混合物(质量比为 2:1),在 pH ∼ 9.0 的条件下,1.0 × 10-4 mol/L 异丁基黄原酸钠(SIBX)只能浮出 ∼ 22 % 的黄铁矿,而在额外添加 70 mg/L SLS 后,黄铁矿的可浮性得到恢复,回收率达到 ∼ 90 %。通过原位原子力显微镜(AFM)、接触角、ZETA电位、傅里叶变换红外光谱仪(FTIR)和光学显微镜推断,SIBX与黄铁矿的强亲和力使其取代了黄铁矿表面大部分预聚的SLS,从而恢复了黄铁矿的疏水性,进一步降低了ZETA电位。此外,SLS 通过其羧基和酚羟基附着在蛇纹石带正电的镁位点上,降低了蛇纹石的疏水性和 Zeta 电位。与软碱 S 原子/阴离子相比,硬碱 O 原子/阴离子对硬酸镁阳离子的亲和力要强得多,而 SIBX 几乎无法取代蛇纹石表面吸附的 SLS。因此,吸附了 SLS 的蛇纹石与 SIBX 疏水化黄铁矿之间的静电排斥力增强,疏水吸引力减弱,从而避免了它们的聚集。SLS 和 SIBX 的结合实现了黄铁矿与蛇纹石的选择性浮选分离。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Surfaces and Interfaces
Chemistry-General Chemistry
CiteScore
8.50
自引率
6.50%
发文量
753
审稿时长
35 days
期刊介绍:
The aim of the journal is to provide a respectful outlet for ''sound science'' papers in all research areas on surfaces and interfaces. We define sound science papers as papers that describe new and well-executed research, but that do not necessarily provide brand new insights or are merely a description of research results.
Surfaces and Interfaces publishes research papers in all fields of surface science which may not always find the right home on first submission to our Elsevier sister journals (Applied Surface, Surface and Coatings Technology, Thin Solid Films)
文献相关原料
公司名称
产品信息
阿拉丁
SLS
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


