Corrosion of iron in liquid uranium hexafluoride at 80 °C. Part I: Normal and abnormal experimental kinetics
IF 1.7
4区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
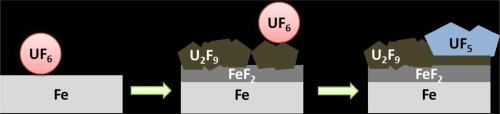
The results reported here are the first on pure iron corrosion in liquid UF6 at 80 °C. Two kinetic behaviours have been observed: one led to micrometric scales (from micron to hundreds of microns after several months) and the other one to hundreds of nanometers for several months. The higher corrosion kinetics resulted in the presence of impurities such as NOxF complexes formed by interaction between the medium and the reactor material. These NOxF catalysed the corrosion reaction leading to a corrosion mechanism controlled by the cathodic reaction rate. Effect of impurities in UF6 coming from experimental conditions or nature of uranium ore should then be systematically and carefully checked. Whatever the corrosion kinetics and the presence of impurities, the nature of the layer was identical: a duplex fluoride scale composed of an iron rich layer, FeF2, and a uranium rich layer evolving over time from U2F9 to UF5.
铁在 80 °C 的液态六氟化铀中的腐蚀。第一部分:正常和异常实验动力学
本文报告的结果是首次对 80 °C 下液态六氟化铀中的纯铁腐蚀进行研究。观察到两种动力学行为:一种是微米尺度(几个月后从微米到几百微米),另一种是几个月后达到几百纳米。较高的腐蚀动力学产生于杂质的存在,如介质和反应器材料之间相互作用形成的 NOxF 复合物。这些 NOxF 催化了腐蚀反应,形成了一种由阴极反应速率控制的腐蚀机制。因此,应系统、仔细地检查实验条件或铀矿石性质对六氟化铀中杂质的影响。无论腐蚀动力学如何以及是否存在杂质,腐蚀层的性质都是相同的:由富铁层 FeF2 和随时间从 U2F9 演化为 UF5 的富铀层组成的双相氟化物鳞片。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Fluorine Chemistry
化学-无机化学与核化学
CiteScore
3.80
自引率
10.50%
发文量
99
审稿时长
33 days
期刊介绍:
The Journal of Fluorine Chemistry contains reviews, original papers and short communications. The journal covers all aspects of pure and applied research on the chemistry as well as on the applications of fluorine, and of compounds or materials where fluorine exercises significant effects. This can include all chemistry research areas (inorganic, organic, organometallic, macromolecular and physical chemistry) but also includes papers on biological/biochemical related aspects of Fluorine chemistry as well as medicinal, agrochemical and pharmacological research. The Journal of Fluorine Chemistry also publishes environmental and industrial papers dealing with aspects of Fluorine chemistry on energy and material sciences. Preparative and physico-chemical investigations as well as theoretical, structural and mechanistic aspects are covered. The Journal, however, does not accept work of purely routine nature.
For reviews and special issues on particular topics of fluorine chemistry or from selected symposia, please contact the Regional Editors for further details.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


