Unlocking therapeutic potential of siRNA-based drug delivery system for treatment of Alzheimer's disease
IF 4.5
3区 医学
Q1 PHARMACOLOGY & PHARMACY
Journal of Drug Delivery Science and Technology
Pub Date : 2024-11-15
DOI:10.1016/j.jddst.2024.106413
引用次数: 0
Abstract
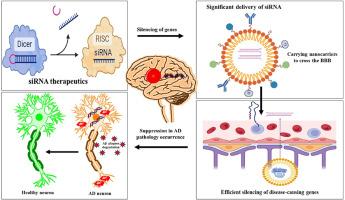
Alzheimer's disease (AD) is a progressive neurodegenerative disorder marked by the accumulation of amyloid-beta (Aβ) plaques and tau tangles, leading to cognitive decline and neuronal loss. While current treatments mainly provide symptomatic relief, they do not target the underlying molecular causes of the disease. In recent years, RNA interference (RNAi), particularly small interfering RNA (siRNA), has emerged as a promising therapeutic approach for AD. siRNA works by selectively silencing genes responsible for disease processes, degrading messenger RNA (mRNA) and preventing protein synthesis. In AD, siRNA is used to target key pathological factors, such as beta-secretase 1 (BACE1), tau protein, and pro-inflammatory cytokines, to reduce Aβ production, preventing tau hyperphosphorylation, and mitigating neuroinflammation. Preclinical studies in animal models have shown promising results, including significant reductions in Aβ levels and improved cognitive function following siRNA delivery. However, several challenges remain in translating siRNA-based therapies to clinical use, including efficient delivery across the blood-brain barrier (BBB), minimizing off-target effects, and ensuring prolonged gene silencing with minimal immune activation. To overcome these barriers, innovative delivery methods are being explored, such as lipid nanoparticles (LNPs), exosomes, and viral vectors, which aim to improve the stability, specificity, and efficiency of siRNA therapies. Despite these challenges, recent advances in siRNA design and delivery technologies hold great promise, offering a potential new approach to modifying the course of AD and providing hope for more effective, disease-modifying treatments.
挖掘基于 siRNA 的给药系统治疗阿尔茨海默病的潜力
阿尔茨海默病(AD)是一种进行性神经退行性疾病,以淀粉样β(Aβ)斑块和tau缠结的累积为特征,导致认知能力下降和神经元丧失。目前的治疗方法主要是缓解症状,但并不针对该疾病的分子病因。近年来,RNA 干扰(RNAi),尤其是小干扰 RNA(siRNA),已成为一种治疗注意力缺失症的有前途的方法。siRNA 通过选择性沉默导致疾病进程的基因、降解信使 RNA(mRNA)和阻止蛋白质合成来发挥作用。在注意力缺失症中,siRNA 被用于靶向关键病理因子,如 beta-secretase 1 (BACE1)、tau 蛋白和促炎细胞因子,以减少 Aβ 的产生,防止 tau 过度磷酸化,减轻神经炎症。在动物模型中进行的临床前研究已显示出良好的效果,包括在施用 siRNA 后,Aβ 水平明显降低,认知功能得到改善。然而,将基于 siRNA 的疗法转化为临床应用仍面临着一些挑战,包括通过血脑屏障(BBB)的高效递送、最大限度地减少脱靶效应以及确保在最小的免疫激活下延长基因沉默时间。为了克服这些障碍,人们正在探索创新的递送方法,如脂质纳米颗粒(LNPs)、外泌体和病毒载体,旨在提高 siRNA 疗法的稳定性、特异性和效率。尽管存在这些挑战,但 siRNA 设计和递送技术的最新进展前景广阔,为改变 AD 病程提供了一种潜在的新方法,并为更有效的疾病改变疗法带来了希望。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.00
自引率
8.00%
发文量
879
审稿时长
94 days
期刊介绍:
The Journal of Drug Delivery Science and Technology is an international journal devoted to drug delivery and pharmaceutical technology. The journal covers all innovative aspects of all pharmaceutical dosage forms and the most advanced research on controlled release, bioavailability and drug absorption, nanomedicines, gene delivery, tissue engineering, etc. Hot topics, related to manufacturing processes and quality control, are also welcomed.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


