Direct synthesis of dimethyl carbonate from methanol and carbon dioxide over Co-Ce-Zr ternary metal solid solution
IF 7.2
2区 工程技术
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
To investigate highly performance catalysts for the direct synthesis of dimethyl carbonate (DMC) from carbon dioxide (CO2) and methanol, a Co0.02/Ce0.7Zr0.3O2 ternary metal solid solution nanoparticle catalyst was synthesized, demonstrating superior performance with a DMC yield of 3.86 mmol g−1 and selectivity of 100 % at 7 MPa and 140 °C. A series of characterizations further validated the successful incorporation of cobalt and zirconium into the crystal lattice of CeO2, resulting in an increased number of acid-base sites on its surface and a rise in oxygen vacancy content from 10.1 % to 28.7 %. The density functional theory (DFT) calculation results further corroborated the experimental findings, indicating that the doping of cobalt and zirconium ions significantly reduced the formation energy of oxygen vacancies on the catalyst surface from 2.53 to −1.38 eV, while concurrently decreasing the adsorption energy of CO2 from −0.33 to −1.74 eV. Additionally, charge calculation results revealed that oxygen vacancies functioned as Lewis acid sites, whereas lattice oxygen atoms served as Lewis base sites, facilitating the cooperative activation of CO2. The results may provide a new approach for designing and improving CeO2-based catalysts for CO2 activation.
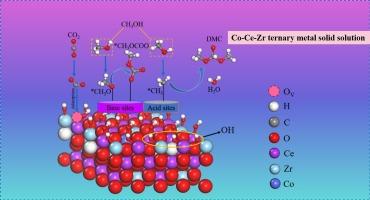
在 Co-Ce-Zr 三元金属固溶体上以甲醇和二氧化碳为原料直接合成碳酸二甲酯
为了研究从二氧化碳(CO2)和甲醇直接合成碳酸二甲酯(DMC)的高性能催化剂,我们合成了一种 Co0.02/Ce0.7Zr0.3O2 三元金属固溶体纳米粒子催化剂,该催化剂性能优越,在 7 兆帕和 140 摄氏度条件下,DMC 产率为 3.86 mmol g-1,选择性为 100%。一系列表征进一步验证了钴和锆成功地融入 CeO2 的晶格,从而增加了其表面酸碱位点的数量,氧空位含量也从 10.1% 上升到 28.7%。密度泛函理论(DFT)计算结果进一步证实了实验结果,表明钴离子和锆离子的掺入显著降低了催化剂表面氧空位的形成能,从 2.53 eV 降至 -1.38 eV,同时二氧化碳的吸附能也从 -0.33 eV 降至 -1.74 eV。此外,电荷计算的结果表明,氧空位作为路易斯酸位,而晶格氧原子作为路易斯碱位,促进了二氧化碳的协同活化。这些结果为设计和改进基于 CeO2 的二氧化碳活化催化剂提供了一种新方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fuel Processing Technology
工程技术-工程:化工
CiteScore
13.20
自引率
9.30%
发文量
398
审稿时长
26 days
期刊介绍:
Fuel Processing Technology (FPT) deals with the scientific and technological aspects of converting fossil and renewable resources to clean fuels, value-added chemicals, fuel-related advanced carbon materials and by-products. In addition to the traditional non-nuclear fossil fuels, biomass and wastes, papers on the integration of renewables such as solar and wind energy and energy storage into the fuel processing processes, as well as papers on the production and conversion of non-carbon-containing fuels such as hydrogen and ammonia, are also welcome. While chemical conversion is emphasized, papers on advanced physical conversion processes are also considered for publication in FPT. Papers on the fundamental aspects of fuel structure and properties will also be considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


