Non-isothermal kinetic analysis of phase transformations in Fe-Co-V-Mo semi-hard magnetic alloy
IF 3.1
2区 化学
Q2 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
In this study, the non-isothermal kinetics and phase transformations within the Fe-Co-V-Mo alloy were investigated. Four distinct transformations were identified: disorder → order (T1), first-stage polymorphic (T2), order → disorder (T3), and second-stage polymorphic (T4). The activation energy (E) for each transformation was determined using isoconversional methods. Fitting model were employed to calculate the kinetic triplets, confirming that all transformations follow the Avrami model. The Johnson-Mehl-Avrami (JMA) and Šesták-Berggren (SB) models were used to determine other kinetic parameters (n, M, and N). The findings suggest that the growth mechanisms for transformations T3 and T4 are interface-controlled, whereas transformation T2 is diffusion-controlled. Consequently, the A3/2 and A3 mechanisms were identified as predominant mechanisms for transformations T2 and T4, respectively. Additionally, transformation T3 follows the A3 mechanism at heating rates of 10 and 20 K/min, and the A2 mechanism at 30 K/min. Kinetic analysis revealed that the addition of Mo in Fe-Co-V alloys, acting as a ferrite (α) stabilizer, decreases the onset temperatures of transformations T1 and T3. Conversely, it increases those of transformations T2 and T4. Furthermore, Mo influences the reduction of E associated with transformation T3.
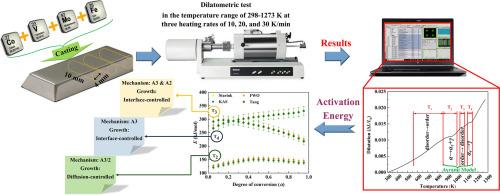
Fe-Co-V-Mo半硬磁性合金相变的非等温动力学分析
本研究调查了铁-铜-钒-钼合金的非等温动力学和相变。确定了四种不同的转变:无序→有序(T1)、第一阶段多态(T2)、有序→无序(T3)和第二阶段多态(T4)。每种转化的活化能(E)都是用等转化法测定的。采用拟合模型计算动力学三元组,证实所有转化都遵循阿夫拉米模型。约翰逊-梅尔-阿夫拉米(JMA)和Šesták-Berggren(SB)模型用于确定其他动力学参数(n、M 和 N)。研究结果表明,转化 T3 和 T4 的生长机制由界面控制,而转化 T2 则由扩散控制。因此,A3/2 和 A3 机制分别被确定为转化 T2 和 T4 的主要机制。此外,在加热速率为 10 和 20 K/min 时,转化 T3 遵循 A3 机制,而在 30 K/min 时则遵循 A2 机制。动力学分析表明,在铁-铜-钒合金中添加钼作为铁素体(α)稳定剂,会降低转化 T1 和 T3 的起始温度。反之,则会提高 T2 和 T4 转变的起始温度。此外,钼还会影响与转变 T3 相关的 E 值的降低。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Thermochimica Acta
化学-分析化学
CiteScore
6.50
自引率
8.60%
发文量
210
审稿时长
40 days
期刊介绍:
Thermochimica Acta publishes original research contributions covering all aspects of thermoanalytical and calorimetric methods and their application to experimental chemistry, physics, biology and engineering. The journal aims to span the whole range from fundamental research to practical application.
The journal focuses on the research that advances physical and analytical science of thermal phenomena. Therefore, the manuscripts are expected to provide important insights into the thermal phenomena studied or to propose significant improvements of analytical or computational techniques employed in thermal studies. Manuscripts that report the results of routine thermal measurements are not suitable for publication in Thermochimica Acta.
The journal particularly welcomes papers from newly emerging areas as well as from the traditional strength areas:
- New and improved instrumentation and methods
- Thermal properties and behavior of materials
- Kinetics of thermally stimulated processes
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


