Effects of Cu(OH)F nanoparticles on the thermal oxidation and ignition characteristics of micron-sized Al powder
IF 3.1
2区 化学
Q2 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
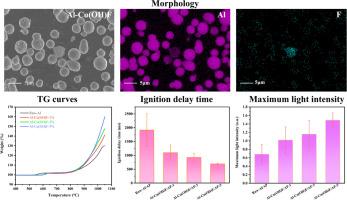
In this study, Cu(OH)F nanoparticles are prepared through a simple hydrothermal reaction and their effects on the thermal oxidation and ignition characteristics of micron-sized Al powder (μ-Al) are explored for the first time. Thermal analysis in air atmosphere (50∼1050 °C) shows that compared to raw μ-Al, the normalized weight gain of Al in Al-Cu(OH)F increases by about 36.9%, 64.8%, and 106.3% when the added Cu(OH)F is 1 wt%, 3 wt%, and 5 wt%, respectively. After mixed with NH4ClO4, electric ignition and open-air combustion tests show shortened ignition delay time (by 42.6%∼63.8%) and increased maximum light intensity (by 48.9%∼117.8%) for Al-Cu(OH)F. The effects of Cu(OH)F result from its decomposition products, namely, HF and CuO. HF reacts with alumina shell to form AlF3, which sublimes at high temperature and thus exposes Al core instantly, while CuO can react with Al core to release heat, further facilitating the thermal oxidation and ignition processes.
纳米 Cu(OH)F 粒子对微米级铝粉热氧化和点火特性的影响
本研究通过简单的水热反应制备了 Cu(OH)F 纳米粒子,并首次探讨了它们对微米级铝粉(μ-Al)的热氧化和点火特性的影响。空气气氛(50∼1050 °C)下的热分析表明,与原始μ-Al 相比,当添加的 Cu(OH)F 为 1 wt%、3 wt% 和 5 wt% 时,Al-Cu(OH)F 中 Al 的归一化增重分别增加了约 36.9%、64.8% 和 106.3%。与 NH4ClO4 混合后,电点火和露天燃烧试验表明,Al-Cu(OH)F 的点火延迟时间缩短(42.6%∼63.8%),最大光强增加(48.9%∼117.8%)。Cu(OH)F 的影响来自其分解产物,即 HF 和 CuO。HF 与氧化铝外壳反应生成 AlF3,后者在高温下发生升华,从而使铝芯瞬间暴露出来,而 CuO 可与铝芯反应释放热量,进一步促进热氧化和点火过程。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Thermochimica Acta
化学-分析化学
CiteScore
6.50
自引率
8.60%
发文量
210
审稿时长
40 days
期刊介绍:
Thermochimica Acta publishes original research contributions covering all aspects of thermoanalytical and calorimetric methods and their application to experimental chemistry, physics, biology and engineering. The journal aims to span the whole range from fundamental research to practical application.
The journal focuses on the research that advances physical and analytical science of thermal phenomena. Therefore, the manuscripts are expected to provide important insights into the thermal phenomena studied or to propose significant improvements of analytical or computational techniques employed in thermal studies. Manuscripts that report the results of routine thermal measurements are not suitable for publication in Thermochimica Acta.
The journal particularly welcomes papers from newly emerging areas as well as from the traditional strength areas:
- New and improved instrumentation and methods
- Thermal properties and behavior of materials
- Kinetics of thermally stimulated processes
文献相关原料
公司名称
产品信息
阿拉丁
Cu(NO3)2·3H2O
阿拉丁
Urea
阿拉丁
NaF
阿拉丁
N-methylpyrrolidone (NMP)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


