Electrosorption of alkaline earth metal ions onto activated carbon as affected by pH and applied potential (pE)
IF 8.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
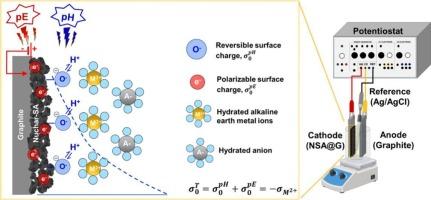
The electrosorption of alkaline earth metal ions from aqueous solution was studied using a graphite supported activated carbon electrode (NSA@G). Zeta potential measurement revealed a low of 3.0 for the NSA electrode, suggesting a negatively charged L-type carbon surface. The electrosorption behavior of Ca2+ ion followed the Langmuir adsorption isotherm and pseudo-first-order rate law. The initial Ca2+ ion concentration, solution pH, and applied potential affected the electrosorption capacity. Results showed that both reversible surface charge (regulated by the potential determining ions, i.e., H+ and OH−, or pH) and polarizable surface charge (controlled by the applied potential, or pE) contributed to the overall Ca2+ ion removal process. The contribution of reversible and polarizable surface charge varied with pH and pE, respectively. Specifically, the reversible surface charge played a more significant role in Ca2+ electrosorption at high pH value and low pE (at 60–83 % of the total Ca2+ uptake), while the polarizable surface charge dominated at low pH and high pE (at 60–62 % of the total Ca2+ uptake). Factors, such as ionic radius, hydration ratio, and hydration enthalpy, significantly affected the electrosorption capacity of divalent alkaline earth metals, i.e., Ca2+, Mg2+, Sr2+, and Ba2+, over NSA@G electrode.
Novelty
This work elucidated the mechanisms of electrode charging and Ca2+ ion uptake via electrosorption. Previous research often attributed ion electrosorption capacity solely to the surface charge derived from a polarizable electrode. In addition to polarizable surface charge, which is controlled by the applied potential (or pE), this study demonstrated that reversible surface charge, regulated by the potential determining ions, i.e., H+ and OH− ions (or pH), also played a significant role in total Ca2+ ion removal. The novelty of this work lies in quantifying the contribution of reversible and polarizable surface charge to overall Ca2+ ion electrosorption. Furthermore, this study investigated the factors affecting the electrosorption behavior of alkaline earth metals, i.e., Mg2+, Ca2+, Sr2+, and Ba2+. A rational approach to predicting electrosorption performance is also proposed, using capacitance characterization from cyclic voltammetry measurements based on Lipmann's electrocapillarity theory.
碱土金属离子在活性炭上的电吸附受 pH 值和外加电位 (pE) 的影响
研究人员使用石墨支撑活性炭电极(NSA@G)对水溶液中的碱土金属离子进行了电吸附。Zeta 电位测量显示,NSA 电极的 pHzpc 值较低,为 3.0,这表明其表面是带负电荷的 L 型碳。Ca2+ 离子的电吸附行为遵循 Langmuir 吸附等温线和伪一阶速率定律。初始 Ca2+ 离子浓度、溶液 pH 值和外加电位都会影响电吸附容量。结果表明,可逆表面电荷(由决定电位的离子,即 H+ 和 OH- 或 pH 值调节)和可极化表面电荷(由外加电位或 pE 控制)对整个 Ca2+ 离子去除过程都有贡献。可逆表面电荷和可极化表面电荷的贡献分别随 pH 值和 pE 值的变化而变化。具体来说,在高 pH 值和低 pE 条件下,可逆表面电荷在 Ca2+ 电吸附过程中起着更重要的作用(占总 Ca2+ 吸收量的 60-83%),而在低 pH 值和高 pE 条件下,可极化表面电荷则占主导地位(占总 Ca2+ 吸收量的 60-62%)。离子半径、水合比和水合焓等因素显著影响了 NSA@G 电极对二价碱土金属(即 Ca2+、Mg2+、Sr2+ 和 Ba2+)的电吸附能力。以往的研究通常将离子电吸附能力完全归因于可极化电极产生的表面电荷。除了由外加电位(或 pE)控制的可极化表面电荷外,本研究还证明了由电位决定离子(即 H+ 和 OH- 离子(或 pH 值))调节的可逆表面电荷在 Ca2+ 离子的总去除中也发挥了重要作用。这项工作的新颖之处在于量化了可逆和可极化表面电荷对整个 Ca2+ 离子电吸附的贡献。此外,这项研究还调查了影响碱土金属(即 Mg2+、Ca2+、Sr2+ 和 Ba2+)电吸附行为的因素。研究还提出了一种预测电吸附性能的合理方法,即利用基于 Lipmann 电消旋理论的循环伏安法测量得出的电容特性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Desalination
工程技术-工程:化工
CiteScore
14.60
自引率
20.20%
发文量
619
审稿时长
41 days
期刊介绍:
Desalination is a scholarly journal that focuses on the field of desalination materials, processes, and associated technologies. It encompasses a wide range of disciplines and aims to publish exceptional papers in this area.
The journal invites submissions that explicitly revolve around water desalting and its applications to various sources such as seawater, groundwater, and wastewater. It particularly encourages research on diverse desalination methods including thermal, membrane, sorption, and hybrid processes.
By providing a platform for innovative studies, Desalination aims to advance the understanding and development of desalination technologies, promoting sustainable solutions for water scarcity challenges.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


