Ultrasound-targeted sirolimus-loaded microbubbles improves acute rejection of heart transplantation in rats by inhibiting TGF-β1-Smad signaling pathway, promoting autophagy and reducing inflammation
IF 5.2
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Acute rejection (AR) remains a pivotal complication and leading cause of mortality within the first year following heart transplantation (HT). In this study, we assessed the impact of ultrasound-targeted microbubbles loaded with sirolimus (SIR-MBs) on AR in a rat HT model and delved into the underlying mechanisms. We established a rat abdominal ectopic HT model, which was stratified into three groups receiveing the PBS, SIR-MBs + ultrasound-targeted microbubble destruction (UTMD), and sirolimus, respectively. The protective effects of each treatments on survival rate, inflammatory response, autophagy and TGF-β1-Smad signaling pathway-related proteins were evaluted. Additionally, rescue experiment was performed via adding the autophagy inhibitor or TGF-β1 agonist in combination therapy. UTMD combined SIR-MBs mediated 15-fold higher local drug concentration compared to direct sirolimus administration. The infiltration of inflammatory cells in the transplanted hearts indicated that SIR-MBs combined with UTMD were effective in mitigating the inflammatory response, achieving levels significantly lower than those observed in the sirolimus group. Furthermore, after SIR-MBs combined with UTMD treatment, the expression levels of TGF-β1-Smad signaling pathway-related proteins in heart tissues also showed a significant decrease compared to the model control group. Conversely, the expressions of autophagy proteins LC3-II, Beclin-1 and β-arrestin showed an up-regulated trend. Rescue experiments also revealed that the enhancement in survival trends was markedly suppressed following the administration of CsA or SRI-011381, respectively. Collectively, our findings suggest that SIR-MBs combined with UTMD augment the local treatment efficacy for AR in rat HT models by inhibiting the TGF-β1-Smad signaling pathway, promoting autophagy, and alleviating inflammation.
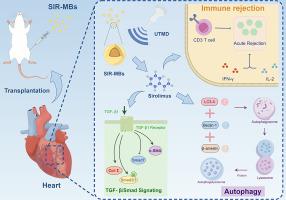
超声靶向西罗莫司负载微气泡通过抑制 TGF-β1-Smad 信号通路、促进自噬和减轻炎症,改善大鼠心脏移植的急性排斥反应
急性排斥反应(AR)仍然是心脏移植(HT)后第一年内的重要并发症和主要致死原因。在这项研究中,我们评估了装载西罗莫司的超声靶向微泡(SIR-MBs)对大鼠心脏移植模型中急性排斥反应的影响,并深入研究了其潜在机制。我们建立了大鼠腹部异位 HT 模型,并将其分为三组,分别接受 PBS、SIR-MBs + 超声靶向微泡破坏(UTMD)和西罗莫司治疗。评估各处理对存活率、炎症反应、自噬和 TGF-β1-Smad 信号通路相关蛋白的保护作用。此外,还通过在联合治疗中加入自噬抑制剂或 TGF-β1 激动剂进行了挽救实验。UTMD联合SIR-MBs介导的局部药物浓度比直接服用西罗莫司高15倍。移植心脏中炎症细胞的浸润情况表明,SIR-MBs 与 UTMD 联用能有效减轻炎症反应,其水平明显低于西罗莫司组。此外,SIR-MBs联合UTMD治疗后,心脏组织中TGF-β1-Smad信号通路相关蛋白的表达水平也比模型对照组显著下降。相反,自噬蛋白LC3-II、Beclin-1和β-arrestin的表达呈上升趋势。挽救实验还发现,分别给予 CsA 或 SRI-011381 后,存活趋势的增强被明显抑制。总之,我们的研究结果表明,SIR-MBs 与UTMD 联合使用可通过抑制 TGF-β1-Smad 信号通路、促进自噬和缓解炎症来增强大鼠 HT 模型中 AR 的局部疗效。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

International Journal of Pharmaceutics: X
Pharmacology, Toxicology and Pharmaceutics-Pharmaceutical Science
CiteScore
6.60
自引率
0.00%
发文量
32
审稿时长
24 days
期刊介绍:
International Journal of Pharmaceutics: X offers authors with high-quality research who want to publish in a gold open access journal the opportunity to make their work immediately, permanently, and freely accessible.
International Journal of Pharmaceutics: X authors will pay an article publishing charge (APC), have a choice of license options, and retain copyright. Please check the APC here. The journal is indexed in SCOPUS, PUBMED, PMC and DOAJ.
The International Journal of Pharmaceutics is the second most cited journal in the "Pharmacy & Pharmacology" category out of 358 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


