High sodium conductive polymer electrolyte-based nanoclusters in supercapacitor
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
The sodium-ion polymer electrolytes (PEs) spur the development of the high safe solid batteries due to their merits of safety, flexibility, lower interfacial resistance with electrodes, and easy processing. Herein, a rational strategy is developed to construct the PE, which can integrate the excellent sodium ion conductivity with the mechanical performances. This strategy applies the nano-sized metal oxide clusters (MOCs) not only to supply the sodium ions but also to inhibit the polymer crystallization. The released polymer chains, crosslinked via physical crosslinking points (nanoclusters), form a network that provides the electrolyte film with toughness over a wide temperature range. The targeted electrolyte exhibits excellent Na-ion conductivity of 3.8 × 10−4 S cm−1 at room temperature, tensile strength up to 0.74 Mpa and breaking elongation of 23 %. In addition, this PE widens the electrochemical stability window of the aqueous Na-ion supercapacitor up to 2.0 V since the water molecules are effectively confined via the strong interactions among components. Our research on sodium polymer electrolytes combined by nanoclusters and PVA holds significant promise for advancing solid-state sodium supercapacitors— a new generation of high-performance, safe, and cost-effective energy storage solutions.
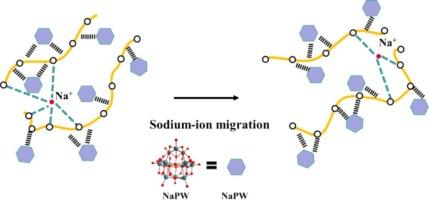
超级电容器中基于导电聚合物电解质的高钠纳米团簇
钠离子聚合物电解质(PE)具有安全、灵活、与电极的界面电阻较低、易于加工等优点,推动了高安全性固体电池的发展。本文提出了一种构建 PE 的合理策略,它能将优异的钠离子传导性与机械性能融为一体。该策略应用了纳米级金属氧化物团簇(MOCs),不仅能提供钠离子,还能抑制聚合物结晶。释放出来的聚合物链通过物理交联点(纳米团簇)交联,形成一个网络,使电解质薄膜在很宽的温度范围内都具有韧性。目标电解质在室温下具有 3.8 × 10-4 S cm-1 的出色钠离子传导性,抗拉强度高达 0.74 兆帕,断裂伸长率为 23%。此外,由于水分子通过各成分之间的强相互作用而被有效地限制,这种钠聚合物电解质拓宽了水性瑙离子超级电容器的电化学稳定性窗口,最高可达 2.0 V。我们关于钠聚合物电解质与纳米团簇和 PVA 结合的研究为推动固态钠超级电容器--新一代高性能、安全、高成本效益的储能解决方案--的发展带来了巨大希望。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


