Injectable microgels containing genetically engineered bacteria for colon cancer therapy through programmed Chemokine expression
IF 8.7
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Chemokines are emerging as important targets for cancer immunotherapy due to their role in regulating immune cell migration and activation within the tumor microenvironment. Effective delivery and sustained presence of chemokines at the tumor site is essential for recruiting and activating immune cells to exert anti-tumor effects. In this study, we report a genetically engineered bacterial cell factory designed for the continuous production of chemokine CCL21 in a controlled manner. To decrease the formation of infusion bodies (IBs) in bacteria, we used thioredoxin (Trx) as the fusion partner and cloned at N-terminal of the target protein. The commonly used promoters, pT7-LacO, pBV220, and pDawn, were employed to explore the influence of various inducers on the expression of CCL21 in bacteria. The engineered bacteria were finally encapsulated within spherical gelatin methacryloyl (GelMA) microgels, which not only maintained bacterial viability but also prolonged their retention in the intestines of mice. As a result, the sustained presence and localized production of CCL21 led to effective suppression of tumor growth.
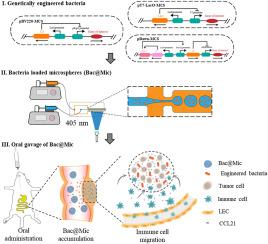
含有基因工程细菌的可注射微凝胶,通过程序化趋化因子表达治疗结肠癌
趋化因子在调节肿瘤微环境中的免疫细胞迁移和激活方面发挥着重要作用,因此正在成为癌症免疫疗法的重要靶点。趋化因子在肿瘤部位的有效传递和持续存在对于招募和激活免疫细胞以发挥抗肿瘤作用至关重要。在这项研究中,我们报告了一种基因工程细菌细胞工厂,其设计目的是以可控方式持续生产趋化因子 CCL21。为了减少细菌中输液体(IB)的形成,我们使用硫代毒素(Trx)作为融合伙伴,并克隆了目标蛋白的 N-末端。我们采用了常用的启动子 pT7-LacO、pBV220 和 pDawn,探讨了各种诱导剂对 CCL21 在细菌中表达的影响。最后,工程细菌被包裹在球形明胶甲基丙烯酰(GelMA)微凝胶中,这不仅保持了细菌的活力,还延长了它们在小鼠肠道中的存留时间。因此,CCL21 的持续存在和局部产生有效抑制了肿瘤的生长。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


