Heterostructure of perovskite coupled graphitic carbon nitride for enhanced photodegradation under visible light
IF 4.3
3区 材料科学
Q2 MATERIALS SCIENCE, COATINGS & FILMS
引用次数: 0
Abstract
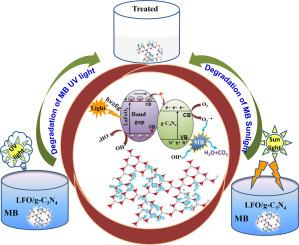
This study aimed to prepare and characterize LaFeO3 nanoparticles loaded graphitic carbon nitride (LFO/g-C3N4) photocatalyst and investigate the degradation of organic pollutants under UV and natural sunlight exposure. It is observed that LFO nanoparticles of an average size of about 67 nm were distributed on g-C3N4 sheets through chemical bonding owing to the formation of a composite. Compared to LFO and g-C3N4, composite has a good absorption ability to harvest more UV and visible light due to its large surface area and pore size. The photocatalytic studies revealed that the g-C3N4/LFO composite exhibits higher catalytic efficiency and stability for methylene blue (MB) degradation under UV and visible light. Under UV light irradiation, the degradation efficiency of LFO, g-C3N4, and g-C3N4/LFO composite in synthetic wastewater was found to be around 50, 80 and 93 % with corresponding rate constants 0.05, 0.02, and 0.1 min−1 in 30 min, respectively. Under natural sunlight, the composite degraded 97 % of MB in 180 min with a rate constant of 0.016 min![]() 1. The higher photocatalytic activity of the composite was attributed to the interfacial charge transfer between LaFeO3 and g-C3N4, which are responsible for effective charge separation in the composite. Further, it has been investigated that singlet oxygen species (1O2) and hydroxyl radicals (•OH) are the main reactive species that contributed considerably to the complete degradation of MB. The nanocomposite was also demonstrated to be a stable catalyst and can be reused without any further modification.
1. The higher photocatalytic activity of the composite was attributed to the interfacial charge transfer between LaFeO3 and g-C3N4, which are responsible for effective charge separation in the composite. Further, it has been investigated that singlet oxygen species (1O2) and hydroxyl radicals (•OH) are the main reactive species that contributed considerably to the complete degradation of MB. The nanocomposite was also demonstrated to be a stable catalyst and can be reused without any further modification.
在可见光下增强光降解的过氧化物耦合氮化石墨异质结构
本研究旨在制备负载氮化石墨碳(LFO/g-C3N4)的 LaFeO3 纳米粒子光催化剂,并研究其在紫外线和自然光照射下降解有机污染物的特性。研究发现,平均尺寸约为 67 nm 的 LFO 纳米粒子通过化学键作用分布在 g-C3N4 片上,形成了一种复合材料。与 LFO 和 g-C3N4 相比,复合材料因其较大的表面积和孔径而具有良好的吸收能力,能吸收更多的紫外线和可见光。光催化研究表明,g-C3N4/LFO 复合材料在紫外光和可见光下降解亚甲基蓝(MB)具有更高的催化效率和稳定性。在紫外光照射下,LFO、g-C3N4 和 g-C3N4/LFO 复合材料在合成废水中的降解效率分别约为 50%、80% 和 93%,30 分钟内的相应速率常数分别为 0.05、0.02 和 0.1 min-1。在自然阳光下,复合材料在 180 分钟内降解了 97% 的甲基溴,速率常数为 0.016 分钟1 。复合材料较高的光催化活性归因于 LaFeO3 和 g-C3N4 之间的界面电荷转移,它们在复合材料中实现了有效的电荷分离。此外,研究还发现,单线态氧(1O2)和羟基自由基(-OH)是主要的反应物种,对甲基溴的完全降解起到了重要作用。研究还证明,这种纳米复合材料是一种稳定的催化剂,可以重复使用,无需进一步改良。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Diamond and Related Materials
工程技术-材料科学:综合
CiteScore
6.00
自引率
14.60%
发文量
702
审稿时长
2.1 months
期刊介绍:
DRM is a leading international journal that publishes new fundamental and applied research on all forms of diamond, the integration of diamond with other advanced materials and development of technologies exploiting diamond. The synthesis, characterization and processing of single crystal diamond, polycrystalline films, nanodiamond powders and heterostructures with other advanced materials are encouraged topics for technical and review articles. In addition to diamond, the journal publishes manuscripts on the synthesis, characterization and application of other related materials including diamond-like carbons, carbon nanotubes, graphene, and boron and carbon nitrides. Articles are sought on the chemical functionalization of diamond and related materials as well as their use in electrochemistry, energy storage and conversion, chemical and biological sensing, imaging, thermal management, photonic and quantum applications, electron emission and electronic devices.
The International Conference on Diamond and Carbon Materials has evolved into the largest and most well attended forum in the field of diamond, providing a forum to showcase the latest results in the science and technology of diamond and other carbon materials such as carbon nanotubes, graphene, and diamond-like carbon. Run annually in association with Diamond and Related Materials the conference provides junior and established researchers the opportunity to exchange the latest results ranging from fundamental physical and chemical concepts to applied research focusing on the next generation carbon-based devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


