De novo myasthenia gravis in a patient with malignant melanoma after concurrent SARS-CoV-2 vaccination and immune checkpoint inhibitor therapy: Case report and literature review
Q3 Neuroscience
引用次数: 0
Abstract
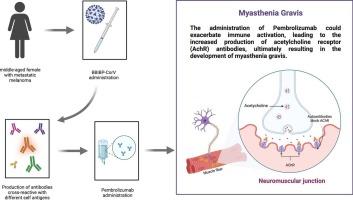
In recent years, the advent and increasingly common use of immune checkpoint inhibitors (ICIs) in cancer treatment have been notable. While ICIs have shown relatively better toxicity profiles compared to traditional chemotherapy agents, they are linked to a unique range of toxicities known as immune-related adverse events (irAEs), stemming from immune system dysregulation. Following the coronavirus disease 2019 (COVID-19) pandemic, cancer patients were universally categorized as the highest priority subgroup for vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), despite being excluded from vaccine trials. The exclusion of cancer patients from vaccine trials has raised concerns within the scientific community about the potential for a hyperactive autoimmune response, which could lead to severe irAEs in patients receiving concurrent ICIs and anti-SARS-CoV-2 vaccines. Retrospective studies have indicated subtle safety concerns for mRNA vaccines in cancer patients who have undergone ICI treatment, with none of these studies encompassing inactivated anti-SARS-CoV-2 vaccines. Here, we present a case of a patient with malignant melanoma who developed fatal myasthenia gravis (MG) following concurrent vaccination with Sinopharm's inactivated COVID-19 vaccine (BBIBP-CorV) and initiation of pembrolizumab. Additionally, we examine current research on the relationship between anti-SARS-CoV-2 vaccination and irAEs in patients treated with ICIs and propose a potential mechanism responsible for the fatal MG in our patient.
一名恶性黑色素瘤患者在同时接种SARS-CoV-2疫苗和接受免疫检查点抑制剂治疗后出现新发肌无力:病例报告和文献综述
近年来,免疫检查点抑制剂(ICIs)在癌症治疗中的出现和日益普遍的应用引人注目。虽然与传统化疗药物相比,免疫检查点抑制剂显示出相对较好的毒性,但它们与一系列独特的毒性有关,即免疫相关不良事件(irAEs),源于免疫系统失调。在 2019 年冠状病毒病(COVID-19)大流行之后,癌症患者被普遍归类为接种严重急性呼吸系统综合征冠状病毒 2(SARS-CoV-2)疫苗的最优先亚群,尽管他们被排除在疫苗试验之外。将癌症患者排除在疫苗试验之外引发了科学界对自身免疫反应亢进可能性的担忧,这可能会导致同时接种 ICIs 和抗 SARS-CoV-2 疫苗的患者出现严重的 irAE。回顾性研究表明,在接受 ICI 治疗的癌症患者中使用 mRNA 疫苗存在微妙的安全性问题,但这些研究均未涉及灭活的抗 SARS-CoV-2 疫苗。在此,我们介绍了一例恶性黑色素瘤患者在同时接种国药集团的 COVID-19 灭活疫苗(BBIBP-CorV)和开始使用 pembrolizumab 后出现致命性肌无力(MG)的病例。此外,我们还考察了目前关于抗SARS-CoV-2疫苗接种与接受ICIs治疗的患者发生irAEs之间关系的研究,并提出了导致我们患者发生致命性MG的潜在机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

eNeurologicalSci
Neuroscience-Neurology
CiteScore
3.50
自引率
0.00%
发文量
45
审稿时长
62 days
期刊介绍:
eNeurologicalSci provides a medium for the prompt publication of original articles in neurology and neuroscience from around the world. eNS places special emphasis on articles that: 1) provide guidance to clinicians around the world (Best Practices, Global Neurology); 2) report cutting-edge science related to neurology (Basic and Translational Sciences); 3) educate readers about relevant and practical clinical outcomes in neurology (Outcomes Research); and 4) summarize or editorialize the current state of the literature (Reviews, Commentaries, and Editorials). eNS accepts most types of manuscripts for consideration including original research papers, short communications, reviews, book reviews, letters to the Editor, opinions and editorials. Topics considered will be from neurology-related fields that are of interest to practicing physicians around the world. Examples include neuromuscular diseases, demyelination, atrophies, dementia, neoplasms, infections, epilepsies, disturbances of consciousness, stroke and cerebral circulation, growth and development, plasticity and intermediary metabolism. The fields covered may include neuroanatomy, neurochemistry, neuroendocrinology, neuroepidemiology, neurogenetics, neuroimmunology, neuroophthalmology, neuropathology, neuropharmacology, neurophysiology, neuropsychology, neuroradiology, neurosurgery, neurooncology, neurotoxicology, restorative neurology, and tropical neurology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


