Electrochemical investigations of decorated graphite felt electrodes with Nafion/TiO2 nanoparticles for vanadium redox flow battery: Improving electro-catalytic characteristics
IF 2.8
Q1 MATERIALS SCIENCE, CERAMICS
引用次数: 0
Abstract
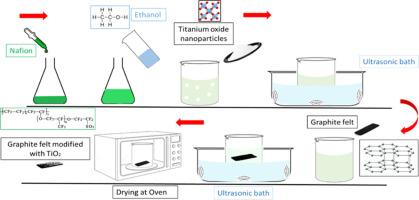
The present study aimed to investigate the effect of Nafion/TiO2 nanoparticles as an electrocatalyst on the electrochemical behavior of graphite felt (GF) electrodes in a vanadium redox flow battery. Various electrochemical tests, including cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), and linear sweep voltammetry (LSV), were conducted to assess the performance of these electrodes at different concentrations of nanoparticles (2–4 g l-1). Additionally, X-ray diffraction, Fourier transform infrared spectroscopy, and field emission scanning electron microscopy were employed to analyze the chemical composition, bonding, and morphology of the decorated electrodes, respectively. The CV results revealed several effects of TiO2 nanoparticles on the GF electrode, such as increased peak intensity and shifted peak sites. As the concentration of nanoparticles increased, the peak intensity rose by up to 44%. Moreover, the potentials of the decorated electrodes shifted towards the favorable side compared to the GF electrode, with changes ranging from 18 to 84%. Overall, the optimal concentration of TiO2 nanoparticles (3 g l-1) exhibited excellent electrode performance, characterized by the highest calculated diffusion coefficient, greater reversibility, enhanced electron transfer kinetics, improved stability, lower over-potential, and the lowest activation energy for redox reactions. EIS results demonstrated a significant decrease in polarization resistance (67.2–70.8%) for the decorated electrodes. Furthermore, LSV measurements indicated that the utilization of nano-electrocatalysts effectively inhibited hydrogen evolution at negative potentials.
用于钒氧化还原液流电池的 Nafion/TiO2 纳米颗粒装饰石墨毡电极的电化学研究:改善电催化特性
本研究旨在探讨 Nafion/TiO2 纳米颗粒作为电催化剂对钒氧化还原液流电池中石墨毡(GF)电极电化学行为的影响。为了评估这些电极在不同纳米颗粒浓度(2-4 g l-1)下的性能,进行了各种电化学测试,包括循环伏安法(CV)、电化学阻抗光谱法(EIS)和线性扫描伏安法(LSV)。此外,还采用 X 射线衍射、傅立叶变换红外光谱和场发射扫描电子显微镜分别分析了装饰电极的化学成分、键合和形态。CV 结果显示了 TiO2 纳米粒子对 GF 电极的一些影响,如峰值强度增加和峰值位移。随着纳米粒子浓度的增加,峰值强度最高上升了 44%。此外,与 GF 电极相比,装饰电极的电位向有利的一侧偏移,变化范围从 18% 到 84%。总体而言,最佳浓度的 TiO2 纳米粒子(3 g l-1)表现出优异的电极性能,其特点是计算扩散系数最高、可逆性更高、电子转移动力学增强、稳定性提高、过电位降低以及氧化还原反应活化能最低。EIS 结果表明,装饰电极的极化电阻显著降低(67.2%-70.8%)。此外,LSV 测量表明,利用纳米电催化剂可有效抑制负电位下的氢演化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Open Ceramics
Materials Science-Materials Chemistry
CiteScore
4.20
自引率
0.00%
发文量
102
审稿时长
67 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


