“On-demand” nanosystem-integrated microneedles for amplified triple therapy against recalcitrant bacteria and biofilm growth
IF 8.7
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Phototherapy has emerged to eradicate recalcitrant bacteria without causing drug resistance, but it is often accompanied by considerable limitations owing to a high tolerance of recalcitrant bacteria to heat and oxidative damage, leading to low efficiency of monotherapy and unwanted side effects. Assuming that employing antimicrobial peptides (AMPs) to disrupt bacterial membranes could reduce bacterial tolerance, a multifunctional “on-demand” nanosystem based on zeolitic imidazolate framework-8 (ZIF-8) with metal ions for intrinsic antibacterial activity was constructed to potently kill methicillin-resistant Staphylococcus aureus (MRSA). Then, microneedles (MNs) were used to transdermally deliver the ZIF-8-based nanosystem for localized skin infection. After MNs insertion, the nanoplatform could specifically deliver the loaded therapeutic components to bacterial infection sites through employing hyaluronic acid (HA) as a capping agent, thus realizing the “on-demand” payload release triggered by excess hyaluronidase secreted by MRSA. The prepared nanosystem and MNs were confirmed to exert an amplified triple therapy originating from membranolytic effect, phototherapy, and ion therapy, thus displaying a powerful bactericidal and MRSA biofilm destruction ability. This intelligent antimicrobial strategy may bring a dawn of hope for eradicating multidrug-resistant bacteria and biofilms.
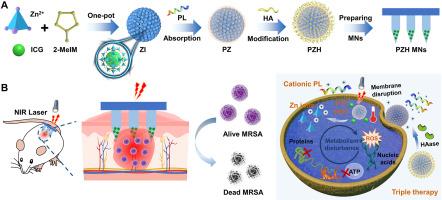
"按需 "集成纳米系统的微针,用于针对顽固细菌和生物膜生长的三重放大疗法
光疗法的出现是为了在不产生耐药性的情况下根除顽固细菌,但由于顽固细菌对热和氧化损伤的耐受性较高,光疗法往往有很大的局限性,导致单一疗法的效率低下和不必要的副作用。假设利用抗菌肽(AMPs)破坏细菌膜可以降低细菌耐受性,研究人员构建了一种基于沸石咪唑酸框架-8(ZIF-8)的多功能 "按需 "纳米系统,其中的金属离子具有内在抗菌活性,可有效杀灭耐甲氧西林金黄色葡萄球菌(MRSA)。然后,利用微针(MNs)透皮递送基于 ZIF-8 的纳米系统,治疗局部皮肤感染。插入微针后,纳米平台可通过使用透明质酸(HA)作为封盖剂,将负载的治疗成分特异性地输送到细菌感染部位,从而实现了由 MRSA 分泌的过量透明质酸酶触发的有效载荷的 "按需 "释放。经证实,制备的纳米系统和 MNs 可发挥源于膜溶解效应、光疗和离子疗法的放大三重疗法,从而显示出强大的杀菌和 MRSA 生物膜破坏能力。这种智能抗菌策略为根除耐多药细菌和生物膜带来了曙光。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


