Anthelmintic activity of selected neolignans and semisynthetic derivatives from Styrax suberifolius
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
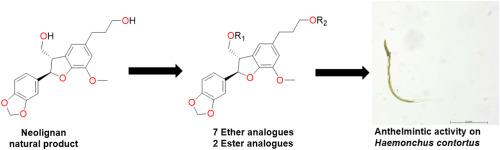
The plant metabolite, equiselignan B (2), isolated from the fruits of Styrax suberifolius was used to generate a semisynthetic library. Extraction and purification studies yielded large quantities (∼70 mg) of 2, a new glycoside, suberifolioside A (1), and the previously described neolignan egonol (3). The planer structure of 1 was established following 1D/2D NMR and MS analyses, and its absolute configuration was determined by X-ray diffraction studies. Acid hydrolysis of 1 yielded an additional amount of scaffold 2 (436 mg) that was subsequently converted into seven new ether derivatives (4–10) and two new ester derivatives (11–12) using commercially available alkyl halides and acyl chlorides, respectively. The chemical structures of the new semisynthetic ether and ester derivatives were assigned by spectroscopic and spectrometric analyses. Subsequently, compounds 1–12 were evaluated for their anthelmintic activity on exsheathed third-stage larvae (xL3s) and fourth-stage larvae (L4s) of Haemonchus contortus (barber's pole worm) – a highly pathogenic parasitic roundworm of ruminant livestock. Ether derivative 5 displayed significant anthelmintic activity against both xL3s and L4s, resulting in a 66–73 % reduction in motility at 50 μM after 168 h (xL3s) or 90 h (L4s) of exposure and IC50 values of 24–30 μM. This derivative also induced curved (Cur), evisceration (Evi) and skinny (Ski) phenotypes in affected larvae. Compounds 1, 9 and 12 also had marked activity against L4s, which indicate that the neolignan structure class warrants further investigation for anthelmintic activity.
从鼠李属植物中提取的部分新木质素及其半合成衍生物的驱虫活性
我们利用从鼠李属(Styrax suberifolius)果实中分离出的植物代谢物马钱子苷 B(2)生成了一个半合成文库。萃取和纯化研究得到了大量(∼70 毫克)2、一种新的苷类--苏木防己苷 A(1)和之前描述过的新木质素 egonol(3)。通过一维/二维核磁共振和质谱分析,确定了 1 的平面结构,并通过 X 射线衍射研究确定了其绝对构型。对 1 进行酸水解后,又得到了一定量的支架 2(436 毫克),随后利用市售的烷基卤化物和酰基氯化物分别将其转化为 7 种新的醚衍生物(4-10)和 2 种新的酯衍生物(11-12)。通过光谱和分光分析,确定了新的半合成醚和酯衍生物的化学结构。随后,评估了化合物 1-12 对反刍家畜的高致病性寄生蛔虫--轮虫(理发杆虫)的出鞘三龄幼虫(xL3s)和四龄幼虫(L4s)的驱虫活性。醚衍生物 5 对 xL3s 和 L4s 均显示出显著的驱虫活性,在接触 50 μM 时,xL3s 和 L4s 在 168 小时(xL3s)或 90 小时(L4s)后的蠕动减少了 66-73 %,IC50 值为 24-30 μM。该衍生物还诱导受影响幼虫出现弯曲(Cur)、剥离(Evi)和瘦小(Ski)表型。化合物 1、9 和 12 对 L4s 也有明显的活性,这表明新木质素结构类化合物的抗蠕虫活性值得进一步研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


