Electrochemical-promoted Mannich-type three-component reaction of benzo-fused cyclic amides using methanol as C1 source instead of formaldehyde
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The three-component N-Mannich reaction is provided in an electrochemical variant. The corresponding N-Mannich base products were obtained by the one-pot method of benzo-fused cyclic amides with secondary amines under a small current of 3 mA using methanol as the carbon source. The reaction is simple to carry out (room temperature and air environment) and requires no supporting electrolytes, using TEMPO as the oxidizing agent. The substrate scope is wide, and the yield is good (39 cases, up to 99 %). CV experiments show that secondary amines are oxidized first at the anode. Moreover, this reaction can be scaled up to the grams.
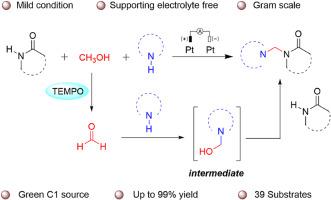
用甲醇代替甲醛作为 C1 源,电化学促进苯并融合环酰胺的曼尼希式三组分反应
三组分 N-Mannich 反应以电化学变体的形式出现。以甲醇为碳源,在 3 mA 的小电流下,通过苯并融合环酰胺与仲胺的一锅法获得了相应的 N-Mannich 碱产物。该反应以 TEMPO 为氧化剂,操作简单(室温和空气环境),无需辅助电解质。底物范围广,产率高(39 例,高达 99%)。CV 实验表明,仲胺首先在阳极被氧化。此外,该反应还可以放大到克级。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


