Optimizing therapeutics: A novel mutual prodrug of ketoprofen and Chlorzoxazone for enhanced efficacy and safety
IF 3.2
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The development of mutual prodrugs signifies a pivotal advancement in the optimization of therapeutic profiles for established pharmaceuticals. This study explores the synthesis and evaluation of an innovative mutual prodrug combining the nonsteroidal anti-inflammatory drug (NSAID) Ketoprofen and the skeletal muscle relaxant Chlorzoxazone. Designed to alleviate gastric irritation inherent to NSAIDs while capitalizing on the synergistic benefits of both agents, the synthesized mutual prodrug was characterized and confirmed through extensive physicochemical and spectroscopic studies. Solubility and partition coefficient analyses indicated heightened lipophilicity, enhancing the prodrug's suitability for oral administration compared to its parent drugs. Additionally, protein binding studies revealed a low binding affinity, suggesting improved bioavailability. Subsequent in vitro hydrolysis studies assessed the prodrug's stability across various pH levels (1.2, 3, 5 and 7.4) simulated gastric and intestinal fluids, plasma and rat liver homogenate, with quantitative evaluation performed by high-performance liquid chromatography. The prodrug remained stable and unhydrolyzed in the stomach after absorption, but underwent rapid cleavage by esterase's in blood and rat liver homogenate, releasing the active parent drugs. Our investigation underscores the transformative potential of mutual prodrug design in optimizing the efficacy and safety profile of combining NSAIDs with muscle relaxants. This approach offers a novel therapeutic strategy that enhances patient outcomes while minimizing adverse effects, paving the way for innovative treatments in managing musculoskeletal conditions and improving overall quality of life.
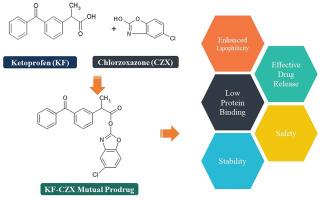
优化疗法:提高疗效和安全性的酮洛芬和氯唑沙宗新型互为原药
互利原药的开发标志着现有药物在优化治疗方案方面取得了关键性进展。本研究探讨了非甾体抗炎药(NSAID)酮洛芬和骨骼肌松弛剂氯唑沙宗的创新互效原药的合成和评估。为了减轻非甾体抗炎药固有的胃刺激症状,同时利用这两种药物的协同作用,我们对合成的互利原药进行了表征,并通过大量的理化和光谱研究加以确认。溶解度和分配系数分析表明,与母药相比,原药具有更高的亲脂性,更适合口服给药。此外,蛋白质结合研究显示,原药的结合亲和力较低,表明生物利用度有所提高。随后的体外水解研究评估了原药在不同 pH 值(1.2、3、5 和 7.4)的模拟胃液、肠液、血浆和大鼠肝匀浆中的稳定性,并通过高效液相色谱法进行了定量评估。原药吸收后在胃中保持稳定且未水解,但在血液和大鼠肝匀浆中会被酯酶迅速裂解,释放出活性母药。我们的研究强调了互利原药设计在优化非甾体抗炎药与肌肉松弛剂联合用药的疗效和安全性方面的变革潜力。这种方法提供了一种新颖的治疗策略,在提高患者疗效的同时将不良反应降至最低,为治疗肌肉骨骼疾病和提高整体生活质量的创新疗法铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


