Synthesis, characterization, insecticidal activity and antibacterial evaluation of some heterocyclic compounds containing 1,2,3-triazoles moiety
IF 3.2
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
A series of 1,2,3-triazole compounds having thymol or paracetamol moieties have been designed via employing ‘click’ chemistry with copper-catalyzed azide-alkyne cycloaddition reactions. Additionally, the insecticidal characterizations of many designed products were perceived with respect to second & fourth of Spodoptera littoralis instar larvae. Numerous insecticides are revealed as novelties. As a result, numerous triazole derivatives were designed chemically to assist as analogues a comprehensive class of insecticides. With LC50 value of 0.321 mg/L for second larvae instar and LC50 = 0.627 mg/L four instar larvae, components 10 display the highest insecticidal bioactivity. This article deliberates how to find novel compounds that could be employed as insecticidal components in the future. A few biological and biochemical properties of the compounds produced in a laboratory environment were also examined. This work also addresses the process of finding new compounds that could be used as insecticidal products in the future. Ultimately, the antibacterial effectiveness of each developed component contrary to both Gram-positive & Gram-negative bacteria was observed.
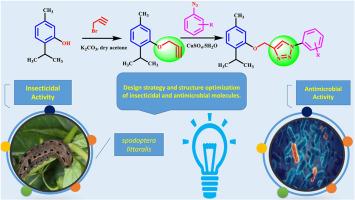
一些含有 1,2,3 三唑分子的杂环化合物的合成、表征、杀虫活性和抗菌评价
通过铜催化叠氮-炔环加成反应的 "点击 "化学,设计出了一系列具有百里酚或扑热息痛分子的 1,2,3-三唑化合物。此外,还对许多设计产品的杀虫特性进行了研究,包括对鞘翅目幼虫的第二、第三和第四龄幼虫的杀虫特性。发现了许多新颖的杀虫剂。因此,我们通过化学方法设计了许多三唑类衍生物,以协助开发一类全面的杀虫剂。成分 10 对第二龄幼虫的半数致死浓度为 0.321 毫克/升,对第四龄幼虫的半数致死浓度为 0.627 毫克/升,显示出最高的杀虫生物活性。本文探讨了如何寻找未来可用作杀虫成分的新型化合物。此外,还研究了在实验室环境中生产的化合物的一些生物和生化特性。这项研究还探讨了寻找未来可用作杀虫产品的新化合物的过程。最后,还观察了所开发的每种成分对革兰氏阳性和阴性细菌的抗菌效果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


