In situ observation and kinetic modeling of the fundamental mechanisms underlying hydrogen sorption in forged Mg–Mg2Ni composites
IF 8.1
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
While Mg–Mg2Ni composites are promising for hydrogen storage, their implementation is hindered by our incomplete understanding of absorption/desorption kinetics. Here, we combine in situ neutron diffraction with kinetic and microstructural analyses to uncover the sorption mechanism of deuterated hydrogen D2 in a Mg–Mg2Ni composite processed by fast forging. Phase transitions upon first absorption are found to be different from subsequent absorptions. The first absorption involves rapid formation of Mg2NiD0.3-x followed by simultaneous formation of MgD2 and Mg2NiD4. Kinetic modeling indicates that surface nucleation of the magnesium hydride is rate-limiting. Subsequent absorptions involve two phases, Mg and Mg2NiD0.3-x, which promote absorption. Kinetic modeling and microstructure analysis indicate that (1) MgD2 nucleation occurs at the Mg–Mg2NiD0.3-x interface and (2) Mg2NiD4 formation is kinetically controlled by deuterium diffusion through the growing Mg2NiD4 plate. In all desorptions, deuterium release starts by rapid decomposition of Mg2NiD4 into Mg2NiD0.3-x, followed by slower MgD2 decomposition.
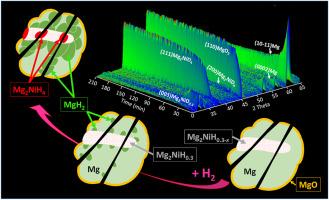
锻造镁-镁-2镍复合材料中氢吸附基本机制的原位观测和动力学建模
虽然镁-镁-Mg2Ni 复合材料在储氢方面大有可为,但由于我们对吸收/解吸动力学的不完全了解,阻碍了其应用。在这里,我们将原位中子衍射与动力学和微结构分析相结合,揭示了通过快速锻造处理的镁-镁-镍复合材料中氘化氢 D2 的吸附机制。研究发现,第一次吸收时的相变不同于随后的吸收。第一次吸收时,Mg2NiD0.3-x 迅速形成,随后 MgD2 和 Mg2NiD4 同时形成。动力学模型表明,氢化镁的表面成核是限制速率的因素。随后的吸收涉及镁和 Mg2NiD0.3-x 两相,它们促进了吸收。动力学建模和微观结构分析表明:(1) MgD2 的成核发生在 Mg-Mg2NiD0.3-x 界面;(2) Mg2NiD4 的形成在动力学上受通过生长的 Mg2NiD4 板的氘扩散的控制。在所有解脱过程中,氘的释放始于 Mg2NiD4 快速分解为 Mg2NiD0.3-x,然后是 MgD2 缓慢分解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


