Self-aldol condensation of acetaldehyde to crotonaldehyde over Lewis acidic metal-incorporated *BEA zeolites
IF 3.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Metal-incorporated *BEA zeolites have shown remarkable catalytic performance in the self-aldol condensation of various aldehydes. The substitution of heteroatom sites can modulate the self-aldol condensation performance of aldehydes, while the underlying mechanisms have been insufficiently explored, with even less attention given to acetaldehyde-related processes. Herein, seven transition metal-incorporated *BEA zeolites were post-synthesized and applied in the self-aldol condensation of acetaldehyde. All the M-BEA zeolites exhibited excellent crotonaldehyde selectivity, exceeding 80 % and most surpassing 85 %, with various acetaldehyde conversions. SEM, XRD, N2 physisorption, FT-IR, UV–Vis, XPS, Py-IR, and NH3-TPD were employed to characterize the physicochemical properties. The M-BEA zeolites predominantly exhibit Lewis acidity, and the amount of Lewis acid with medium strength dominated the acetaldehyde conversion variations. The probe-pulse and TPSR experiments were further employed to investigate the reaction pathways to explain the product distribution on the Zr-BEA zeolite, which demonstrated the highest crotonaldehyde yield. The main side products were long-chain unsaturated aldehydes and cyclic compounds from excessive aldol condensation and cyclization. A reaction network for acetaldehyde conversion on Zr-BEA zeolite was ultimately proposed.
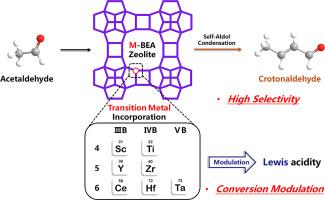
乙醛在路易斯酸性金属掺杂 *BEA 沸石上自醛缩合为巴豆醛
金属掺杂的*BEA沸石在各种醛的自醛缩合过程中表现出显著的催化性能。杂原子位点的取代可以调节醛的自醛缩合性能,但对其潜在机理的探索还不够,对乙醛相关过程的关注更是少之又少。本文对七种过渡金属掺杂的*BEA沸石进行了后合成,并将其应用于乙醛的自醛缩合。所有 M-BEA 沸石都表现出优异的巴豆醛选择性,超过 80%,大多数超过 85%,并具有不同的乙醛转化率。研究人员采用了扫描电镜、X射线衍射、N2物理吸附、傅立叶变换红外光谱、紫外可见光、XPS、Py-IR和NH3-TPD来表征这些沸石的理化性质。M-BEA 沸石主要表现出路易斯酸性,中等强度的路易斯酸量主导了乙醛转化率的变化。探针脉冲和 TPSR 实验进一步研究了反应途径,以解释 Zr-BEA 沸石上的产物分布,该沸石的巴豆醛产率最高。主要的副产物是长链不饱和醛和过量醛醇缩合和环化产生的环状化合物。最终提出了在 Zr-BEA 沸石上进行乙醛转化的反应网络。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


