Novel organocatalytic system of R-bian, R-mian and NHPI for selective oxidation of cumene
IF 4.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The paper describes the influence of a series of bis(arylimino)acenaphthenes (R-bian) and mono(arylimino)acenapthenones (R-mian) in the aerobic oxidation of cumene. The imines facilitate the decomposition of minor impurities of cumene hydroperoxide, thereby acting as catalysts for the oxidation of cumene. The relative reactivity of imines in the oxidation of cumene is correlated with the relative electron-withdrawing ability of the aryl functional groups of CF3-Ph>dpp, tmp. This is demonstrated by the reactivity of dpp-mian, tmp-mian and dpp-bian, tmp-bian<CF3-Ph-bian. A comparable interaction between R-bian and NHPI enhances the solubility of the radical catalyst in cumene and intensifies NHPI-catalyzed oxidation to attain 25–30 % conversion of cumene with dpp-bian and tmp-bian promoters. The reported results present a straightforward organocatalytic system based on the utilization of imines as catalysts and promoters.
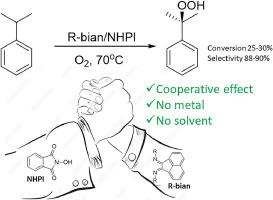
R-卞、R-卞和 NHPI 的新型有机催化系统用于选择性氧化积烯
本文介绍了一系列双(芳基亚氨基)苊(R-bian)和单(芳基亚氨基)苊酮(R-mian)在积烯有氧氧化过程中的影响。这些亚胺能促进过氧化氢中的少量杂质分解,从而起到催化剂的作用。亚胺在积烯氧化过程中的相对反应性与 CF3-Ph>dpp、tmp 的芳基官能团的相对吸电子能力有关。dpp-mian, tmp-mian 和 dpp-bian, tmp-bian<CF3-Ph-bian 的反应性证明了这一点。R-bian 和 NHPI 之间类似的相互作用增强了自由基催化剂在积雪烯中的溶解度,并加强了 NHPI 催化的氧化作用,使 dpp-bian 和 tmp-bian 促进剂对积雪烯的转化率达到 25-30%。所报告的结果展示了一种利用亚胺作为催化剂和促进剂的直接有机催化系统。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


