Modeling the role of Ta-dopant and co-catalytic water for activation of CO2 on anatase TiO2(101)
IF 3.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
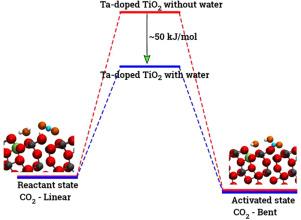
Herein, we have performed DFT+U calculations to investigate the adsorption and activation of CO2 on a Ta-doped anatase TiO2(101) and compared it to that on a pristine anatase TiO2(101). Replacing Ti with a higher valence Ta-dopant results in an excess electron, which localizes on the neighboring Ti atom. We find that there is a slight increase in adsorption energy of CO2 with Ta-doping, however, there is no significant change in the barriers for CO2 activation. Further, presence of a water molecule enhances the adsorption energy and stabilizes the CO2 molecule having both linear and bent configurations (activated). This stabilization is more pronounced for a activated CO2 molecule, which is attributed to the strong H-bonding. Apart from being a reactant, water also acts as a co-catalyst and significantly reduces the barrier for CO2 activation. This is true for both anatase TiO2(101) and Ta-doped anatase TiO2(101). We have also investigated the activation of CO2 via hydrogen and two electron transfer, which constitutes the limiting step in the photochemical reduction of CO2 to CH4. Our calculations show that the barriers for CO2 activation via the two-electron pathway are similar for both anatase TiO2(101) and Ta-doped TiO2(101). However, presence of a water molecule on Ta-doped TiO2(101), not only stabilizes the CO2 molecule but also reduces the barrier for CO2 activation dramatically by acting as a co-catalyst.
模拟掺杂 Ta 和共催化水在锐钛矿二氧化钛(101)上活化 CO2 的作用
在此,我们进行了 DFT+U 计算,以研究二氧化碳在掺杂 Ta 的锐钛矿二氧化钛(101)上的吸附和活化,并与原始锐钛矿二氧化钛(101)上的吸附和活化进行了比较。用更高价的掺杂剂 Ta 取代 Ti 会产生一个过剩电子,该电子定位于邻近的 Ti 原子上。我们发现,掺杂 Ta 后,二氧化碳的吸附能略有增加,但二氧化碳的活化势垒没有显著变化。此外,水分子的存在提高了吸附能,并稳定了具有线性和弯曲构型(活化)的 CO2 分子。这种稳定作用在活化的 CO2 分子中更为明显,这归因于强 H 键。水除了是一种反应物外,还是一种辅助催化剂,能显著降低二氧化碳活化的障碍。锐钛型二氧化钛(101)和掺杂钽的锐钛型二氧化钛(101)都是如此。我们还研究了通过氢和两个电子转移活化 CO2 的情况,这是 CO2 光化学还原为 CH4 的限制步骤。我们的计算表明,锐钛型二氧化钛(101)和掺杂钽的二氧化钛(101)通过双电子途径活化二氧化碳的障碍相似。然而,掺杂钽的二氧化钛(101)上水分子的存在不仅稳定了二氧化碳分子,还通过作为辅助催化剂大大降低了二氧化碳的活化障碍。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


