Additive effect of alkaline earth metal on hydrogen production via catalytic ammonia decomposition over Co/CeO2 catalysts
IF 8.3
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The production of hydrogen from directly catalytic ammonia decomposition shows promise in improving the ammonia viability for engine use. For practical application, it is demanded to investigate additives as well as their impact mechanism on the catalytic properties of transition metal catalysts. Herein, a series of Co/CeO2 catalysts were prepared via a facile precipitation method and the effect of Mg adding was investigated. It is found that addition of 1% Mg decreased the catalytic activity of 5% and 10% Co/CeO2, while increased that of 20% Co/CeO2. Systematic characterizations revealed that Mg promoted the metal-support interaction, which not only benefited the dispersion of Co, but also increased the valence state of Co and enhanced the reducibility. Also, more oxygen vacancies were generated, thus benefiting the reaction process. This work invested new insights into the role of alkaline-earth metal in NH3 decomposition and provided a strategy to modulate the hydrogen–ammonia ratio which is vital for NH3 combustion.
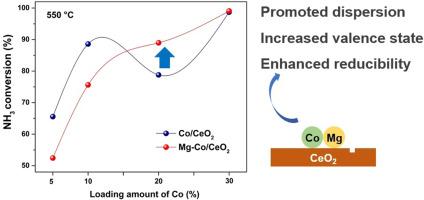
碱土金属对 Co/CeO2 催化剂催化氨分解制氢的添加效应
直接催化氨分解制氢有望提高氨在发动机中的使用率。在实际应用中,需要研究添加剂及其对过渡金属催化剂催化性能的影响机理。本文通过简便沉淀法制备了一系列 Co/CeO2 催化剂,并研究了添加镁的影响。研究发现,添加 1%的镁会降低 5% 和 10% Co/CeO2 的催化活性,而提高 20% Co/CeO2 的催化活性。系统特性分析表明,镁促进了金属与支撑物之间的相互作用,这不仅有利于 Co 的分散,还提高了 Co 的价态,增强了还原性。此外,还产生了更多的氧空位,从而有利于反应过程。这项研究对碱土金属在 NH3 分解中的作用有了新的认识,并为调节对 NH3 燃烧至关重要的氢氨比提供了一种策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


