Greatly boosted H2O2 activity in two-electron water oxidation reaction on Zn-based catalysts by doping engineering
IF 8.3
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
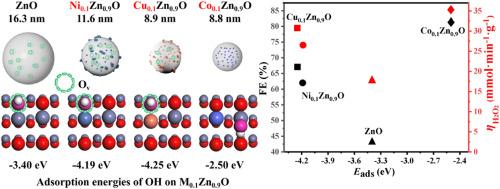
Electrocatalytic production of hydrogen peroxide (H2O2) by two electron water oxidation reaction (2e-WOR) is an environmental process with low cost and devoid of H2O2 storage and transportation. ZnO is one of the important promising 2e-WOR catalysts with relatively high activity and selectivity of H2O2. However, the active sites and the effects of oxygen defects of ZnO remain unknown, which hampers the further improvement in H2O2 formation. To explore the active sites and develop Zn-based catalysts with high performance, Ni, Cu and Co ions are chosen to form M0.1Zn0.9O (M = Ni, Cu and Co) catalysts. Combining the experimental results with density functional theory (DFT) calculations, the active site of 2e-WOR on Zn-based catalysts is first discovered to be the 3-coordinated surface Zn without surface oxygen defects since oxygen defects result in a strong interaction of ·OH with catalyst and then impede the 2e-WOR process. Catalysts with high activity and selectivity for 2e-WOR should have small nano-particle sizes without oxygen defects. Co0.1Zn0.9O is obtained with high activity (35.3 mmol min−1·gcat−1) and selectivity (82%) of H2O2 at 3.2 V vs RHE. This work provides valuable perspectives for designing and developing high-performing catalysts in 2e-WOR reaction.
通过掺杂工程大幅提高 Zn 基催化剂在双电子水氧化反应中的 H2O2 活性
通过双电子水氧化反应(2e-WOR)电催化生产过氧化氢(H2O2)是一种低成本、无需 H2O2 储存和运输的环保工艺。氧化锌是一种重要的有前途的 2e-WOR 催化剂,具有相对较高的活性和 H2O2 选择性。然而,ZnO 的活性位点和氧缺陷的影响仍然未知,这阻碍了 H2O2 生成能力的进一步提高。为了探索活性位点并开发具有高性能的 Zn 基催化剂,我们选择了 Ni、Cu 和 Co 离子来形成 M0.1Zn0.9O(M = Ni、Cu 和 Co)催化剂。将实验结果与密度泛函理论(DFT)计算相结合,首先发现 2e-WOR 在 Zn 基催化剂上的活性位点是没有表面氧缺陷的 3 配位表面 Zn,因为氧缺陷会导致 -OH 与催化剂的强烈相互作用,进而阻碍 2e-WOR 过程。具有高活性和高选择性的 2e-WOR 催化剂应具有无氧缺陷的小纳米颗粒尺寸。Co0.1Zn0.9O 在 3.2 V 对比 RHE 时具有高活性(35.3 mmol min-1-gcat-1)和对 H2O2 的高选择性(82%)。这项工作为设计和开发 2e-WOR 反应中的高性能催化剂提供了宝贵的前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


