Boosting interfacial charge transfer of 2D g-C3N4 by incorporating 0D Ag and 2D metallic NiCo2O4 as dual electron donor and acceptor co-catalysts for photocatalytic hydrogen evolution
IF 8.1
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Efficient light absorption and photoinduced electron transfer from the g-C3N4 (CN) continue to be an ongoing challenge in photocatalytic hydrogen production. Nanodimensional metallic cocatalysts can offer superior electron transport pathways, thereby augmenting photocatalytic activity. In our work metallic NiCo2O4 (NCO) acts as an electron acceptor cocatalyst in a 2D-2D Schottky junction with CN and 0D silver (Ag) functions as a hot electron donor via the localized surface plasmon resonance phenomenon. The novel Ag–CN–NCO nanocomposite was shown to boost visible light absorption while inhibiting charge carrier recombination through optical experiments. The Ag–CN–NCO nanocomposite demonstrated superior photocatalytic activity compared to CN loaded with a single cocatalyst, producing hydrogen at a rate of approximately 2320 μmol/g/h. Additionally, Ag–CN–NCO produced a lower overpotential and almost five times more photocurrent density than CN, as demonstrated by photoelectrochemical studies. This work highlights the development of a novel charge transfer pathway by combining two co-catalysts with different functions and their combined action on the photocatalytic hydrogen production process.
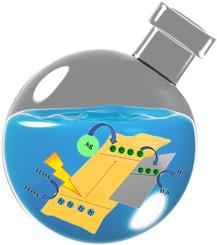
将 0D Ag 和 2D 金属 NiCo2O4 作为光催化氢气进化的双重电子供体和受体助催化剂,促进 2D g-C3N4 的界面电荷转移
g-C3N4 (CN)的高效光吸收和光诱导电子传递仍然是光催化制氢过程中的一项持续挑战。纳米金属茧催化剂可提供出色的电子传输途径,从而提高光催化活性。在我们的研究中,金属镍钴氧化物(NCO)在与氯化萘的 2D-2D 肖特基结中充当电子受体协同催化剂,而 0D 银(Ag)则通过局部表面等离子体共振现象充当热电子供体。光学实验表明,新型 Ag-CN-NCO 纳米复合材料在抑制电荷载流子重组的同时,还能促进可见光的吸收。Ag-CN-NCO 纳米复合材料的光催化活性优于负载单一助催化剂的 CN,产生氢气的速率约为 2320 μmol/g/h。此外,光电化学研究表明,Ag-CN-NCO 产生的过电位更低,光电流密度几乎是 CN 的五倍。这项工作强调了通过结合两种具有不同功能的助催化剂及其对光催化制氢过程的联合作用,开发出一种新型电荷转移途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


