A novel approach for the preparation of furandiamines utilizing biomass platform chemicals as substrates via Gabriel synthesis
IF 3.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Primary diamines are significant chemical intermediates and raw materials for high-added-value chemical products, which are regarded as essential monomers in the synthesis of polyamides and polyurethanes. Primary diamines are also particularly pronounced in the automotive, aerospace, and pharmaceutical fields. Among them, 2,5-bis(aminomethyl)furan (BAF) has garnered grant attention as a highly promising renewable diamine. In this work, a new route for the reduction preparation of BAF using a platform compound 5-chloromethylfurfural (CMF) utilizing the Gabriel method was developed, which reasonably avoids the disadvantage of molecular internal polymerization that often occurs in traditional routes that converting BAF from 5-hydroxymethylfurfural (HMF) and HMF derivatives. Additionally, under mild reaction conditions, this novel route yields BAF efficiently by employing Ni/SiO2 as the catalyst. According to the kinetic analysis the reduction process was proved to be predominant. In addition, the analysis of the mechanism showed that compared to other catalysts, Ni/SiO2 contains more active sites and more hydrogen active components (Ni0), achieving an impressive yield of 82.35 % under mild conditions. This work provides a pioneering method for the preparation of BAF, which is crucial for the development of primary diamine preparation.
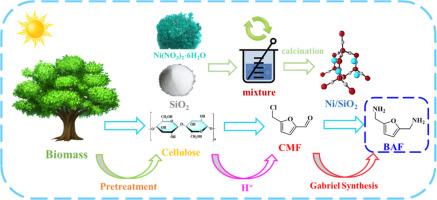
通过加布里埃尔合成法以生物质平台化学品为底物制备呋喃二胺的新方法
原生二胺是重要的化学中间体和高附加值化工产品的原材料,被视为合成聚酰胺和聚氨酯的基本单体。在汽车、航空航天和制药领域,伯胺的作用也尤为突出。其中,2,5-双(氨基甲基)呋喃(BAF)作为一种极具发展前景的可再生二元胺备受关注。在这项工作中,利用加布里埃尔法,开发出了一种利用平台化合物 5-氯甲基糠醛(CMF)还原制备 BAF 的新路线,合理地避免了由 5-羟甲基糠醛(HMF)和 HMF 衍生物转化 BAF 的传统路线中经常出现的分子内部聚合的缺点。此外,在温和的反应条件下,采用 Ni/SiO2 作为催化剂,这条新路线可以高效地生成 BAF。根据动力学分析,还原过程被证明是主要的。此外,机理分析表明,与其他催化剂相比,Ni/SiO2 含有更多的活性位点和更多的氢活性成分(Ni0),在温和条件下的产率高达 82.35%。这项研究为 BAF 的制备提供了一种开创性的方法,这对于初级二胺制备的发展至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


